| CPC C07D 487/04 (2013.01) [A61K 31/428 (2013.01); A61K 31/4439 (2013.01); A61K 31/4709 (2013.01); A61K 31/498 (2013.01); A61K 31/506 (2013.01); A61K 31/53 (2013.01); A61K 31/5377 (2013.01)] | 31 Claims |
|
1. A process for preparing a compound of Formula (I):
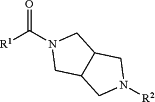 wherein:
R1 is selected from the group consisting of:
A) phenyl substituted or unsubstituted with one or two Ra members, and substituted in the ortho position with Rb, wherein:
Ra is independently selected from the group consisting of —H, halo, —C1-4alkyl, —C1-4alkoxy, and —NO2, wherein two adjacent Ra members may come together to form a six membered aromatic ring;
Rb is a member selected from the group consisting of:
a) halo, —C1-4alkoxy, —C1-4alkyl, —CF3, —OCF3, or —CN;
b) 5-membered heteroaryl ring containing one oxygen or one sulfur members;
c) 5-6 membered heteroaryl ring containing one, two or three nitrogen members, optionally containing one oxygen member, substituted or unsubstituted with halo or —C1-4alkyl; and
d) phenyl substituted or unsubstituted with halo, —CH3, or —CF3;
B) pyridine substituted or unsubstituted with one or two Rc members and substituted with Rd, wherein Rd is positioned adjacent to the point of attachment by R1;
Rc is C1-4alkyl;
Rd is a member selected from the group consisting of:
a) 5-6 membered heteroaryl ring selected from the group consisting of 1H-1,2,3-triazol-1-yl, 2H-1,2,3-triazol-2-yl, 1H-pyrazol-5-yl, 3-methyl-1,2,4-oxadiazol-5-yl, pyridinyl, 3-methyl-pyridin-2-yl, 1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-5-yl), and pyrimidin-2-yl;
b) —CF3, —Br, and —C1-4alkoxy;
C) 5-membered heteroaryl ring selected from the group consisting of 2-methyl-1,3-thiazol-yl, 1H-pyrazol-5-yl, oxazole, isoxazolyl, thiophen-2-yl, and furan-2-yl, each substituted with phenyl substituted or unsubstituted with —F; and
D) 5-13 membered aryl or heteroaryl ring selected from the group consisting of 3-methylfuran-2-yl, 9H-fluorene, quinoline, cinnoline, 3-(1H-pyrrol-1-yl)thiophen-2-yl, 8-[1,2,3]-triazol-2-yl-naphthalen-1-yl, 2,3-dihydro-1,4-benzodioxin-5-yl, 1H-indol-7-yl, 4-fluoronaphthalen-1-yl, and naphthalen-1-yl;
R2 is selected from the group consisting of:
A) 6-membered heteroaryl ring containing two nitrogen members substituted with one or more members independently selected from the group consisting of halo, —C1-4alkyl, —CD3, -D, —C1-4alkoxy, cyclopropyl, morpholin-2-yl, —CO2C1-4alkyl, —CO2H, —CH2OH, —C(O)N(C1-4alkyl)2, —CF3, —CN, —OH, —NO2, —N(C1-4alkyl)2, phenyl, furan-2-yl, thiophen-2-yl, 1H-pyrazol-4-yl, and pyrrolidin-1-yl;
B) pyridine substituted with one or two members independently selected from the group consisting of halo, —C1-4alkyl, —C1-4alkoxy, and —CF3;
C) 9-membered heteroaryl ring selected from the group consisting of benzooxazol-2-yl, 6-fluoro-1,3-benzothiazole, 1,3-benzothiazole, 6-methoxy-1,3-benzothiazole, 6-methyl-1,3-benzothiazole, 6-chloro-benzothiazol-2-yl, and 4-methyl-6,7-dihydro-5H-cyclopenta[d]pyrimidine;
D) 10-membered heteroaryl ring selected from the group consisting of quinoxalin-2-yl, 3-methylquinoxalin-2-yl, 6,7-difluoroquinoxalin-2-yl, 3-(trifluoromethyl)quinoxaline, quinoline, 4-methylquinoline, and 6-fluoroquinazolin-2-yl; and
E) 4-methyl-1,3,5-triazin-2-yl or 2-methylpyrimidin-4(3H)-one,
the process comprising reacting a compound of Formula (I-a):
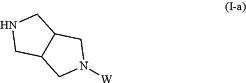 wherein, W is benzyl or BOC, with R1CO2H or R1COCl to form a compound of Formula (I-b):
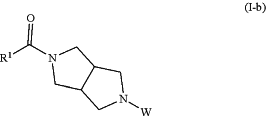 deprotecting the compound of Formula (I-b) to form a compound of formula (I-c):
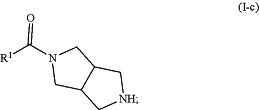 and
reacting the compound of Formula (I-c) with R2Cl to form the compound of Formula (I).
|