| CPC C07D 487/04 (2013.01) [C07D 209/42 (2013.01); C07D 265/36 (2013.01); C07D 417/12 (2013.01); C40B 30/06 (2013.01); C40B 40/14 (2013.01); G16C 20/50 (2019.02)] | 6 Claims |
|
1. A pharmaceutical composition comprising, as an active ingredient, a compound having the general formula:
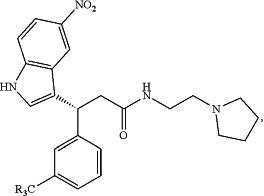 wherein each R may be the same or different and is selected from the group consisting of hydrogen, fluorine, chlorine, bromine, iodine, carboxyl, alkanes, cyclic alkanes, alkenes, cyclic alkenes, alkynes, ketones, aldehydes, carboxylic acids, ethers, esters, amines, amides, monocyclic or polycyclic arene, heteroarenes, phenols, and benzoic acid; and a pharmaceutically acceptable carrier or excipient;
wherein the concentration of the active ingredient is sufficient to inhibit the formation of MCF7 mammospheres in a human tumor, wherein the pharmaceutically acceptable carrier or excipient comprises at least one of sodium alginate, agar powder, laminalia powder, sodium hydrogen carbonate, calcium carbonate, fatty acid esters of polyoxyethylene sorbitan, sodium laurylsulfate, a monoglyceride of stearic acid, and lactose.
|