| CPC C07D 471/04 (2013.01) [A61K 31/437 (2013.01); A61P 11/00 (2018.01); A61P 11/06 (2018.01); C07D 519/00 (2013.01)] | 19 Claims |
|
1. A method for inhibiting a Janus kinase enzyme in a mammal, the method comprising administering to the mammal a compound of formula (I):
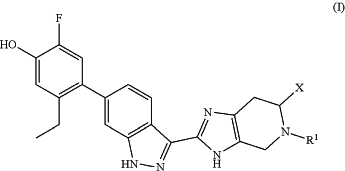 wherein:
R1 is selected from hydrogen, C1-3alkyl, and C3-6cycloalkyl, and X is selected from —C(O)R2 and —CH2R16, or
R1 is selected from —(CH2)2NR20R21 and a 4- to 6-membered heterocyclyl containing one nitrogen atom, wherein the nitrogen atom is optionally substituted with R22, and X is selected from —CH2OR23 and —C(O)OR24,
wherein
R2 is selected from —NR13R14 and —OR15,
R13 and R14 taken together with the nitrogen atom to which they are attached form a 6- or 7-membered monocyclic or bicyclic heterocyclyl containing one additional nitrogen atom, wherein the additional nitrogen atom is substituted with R3 and the heterocyclyl is optionally substituted with one or two R4, or
R13 and R14 taken together with the nitrogen atom to which they are attached form a 5- to 6-membered heterocyclyl, wherein the heterocyclyl is optionally substituted with —NR5R6 and R7, or
R13 and R14 taken together with the nitrogen atom to which they are attached form morpholinyl, or
R13 is R8 and R14 is R9,
R3 is selected from hydrogen, C3-6cycloalkyl, and C1-3alkyl, wherein C1-3alkyl is optionally substituted with —OH or —OC1-3alkyl,
R4 is C1-3alkyl, wherein C1-3alkyl is optionally substituted with —OH,
R5 and R6 are independently C1-3alkyl or R5 and R6 taken together with the nitrogen atom to which they are attached form a 5- or 6-membered heterocyclyl optionally including an oxygen atom,
R7 is C1-3alkyl, optionally substituted with a 5- or 6-membered heterocyclyl containing one nitrogen atom,
R8 is hydrogen or C1-3alkyl,
R9 is —(CH2)2NR10R11 or a 4- to 6-membered heterocyclyl containing one nitrogen atom, wherein the nitrogen atom is substituted with R12,
R10 and R11 are independently C1-3alkyl or R10 and R11 taken together with the nitrogen atom to which they are attached form a 5- or 6-membered heterocyclyl,
R12 is C1-3alkyl or C3-6cycloalkyl, wherein C1-3alkyl is optionally substituted with —OH,
R15 is selected from C1-3alkyl, C3-6cycloalkyl, and a 5- or 6-membered heterocyclyl including one heteroatom selected from nitrogen and oxygen, wherein C1-3alkyl is optionally substituted with —OH or —N(C1-3alkyl)2, and a 5- or 6-membered heterocyclyl is optionally substituted with C1-3alkyl,
R16 is selected from —NR17R18 and —OR19,
R17 and R18 are independently C1-4alkyl or C3-5cycloalkyl or R17 and R18 taken together with the nitrogen atom to which they are attached form a 5- or 6-membered heterocyclyl optionally including an oxygen atom, wherein the heterocyclyl is optionally substituted with C1-3alkyl, and
R19, R20, R21, R22, R23, and R24 are independently selected from hydrogen and C1-3alkyl,
or a pharmaceutically acceptable salt thereof.
|