| CPC C07D 471/04 (2013.01) [C07D 231/56 (2013.01); C07D 235/06 (2013.01); C07D 263/56 (2013.01); C07D 277/64 (2013.01); C07D 333/54 (2013.01); C07D 333/56 (2013.01); C07D 333/58 (2013.01); C07D 409/12 (2013.01); C07D 519/00 (2013.01)] | 32 Claims |
|
1. A compound having structure (I):
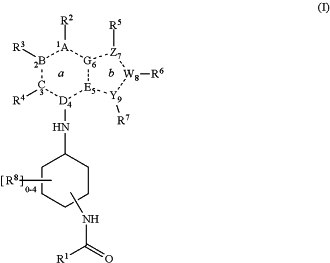 or a pharmaceutically acceptable salt, isomer, hydrate, solvate or isotope thereof, wherein:
R1 is cycloalkyl, aryl, heterocyclyl, —(CH2)nQ, —CHQR, or —CQ(R)2, wherein R1 is optionally substituted with one or more Rq1;
Q is C1-6 alkyl, aryl, cycloalkyl, heterocyclyl, —CH2C(O)OR, —C(O)OR, —C(O)NHR, haloalkyl, —CN, —N(R)2, —N(R)C(O)R, —N(R)C(O)OR, or —N(R)S(O)2R, wherein Q is optionally substituted with one or more Rq2;
Rq1 and Rq2 are independently H, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, aryl, cycloalkyl, heteroaryl, heterocyclyl, —OR, —O(CH2)nR, haloalkoxy, —C(O)OR, —C(O)R, —OC(O)R, halo, haloalkyl, —CN, —N(R)2, —N(R)C(O)R, —N(R)S(O)2R, S(O)2R, —C(H)Q′R, or —(CH2)nQ′ where Q′ is selected from C1-6 alkyl, aryl, cycloalkyl, heterocyclyl, OR′, —C(O)OR′, —OC(O)R′, haloalkyl, —CN, —N(R′)2, —N(R′)C(O)R′, and —N(R′)S(O)2R′;
A is N or CRa;
B is N or CRb;
C is N or CRc;
D is CRd;
E is N or CRe;
G is N or CRg;
W is CRw;
Y is N, S or CRy;
Z is N, CRz, S or O;
each R is independently H, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, alkylamino, —(CH2)nR′, halo, aryl, cycloalkyl, heteroaryl or heterocyclyl, or two R groups taken together with the atom to which they are attached form a carbocyle or heterocycle;
R′ is H, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, haloalkyl, aryl, cycloalkyl, heteroaryl, or heterocyclyl;
Ra is H, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, aryl, cycloalkyl, heteroaryl, heterocyclyl, —OR, —C(O)OR, —OC(O)R, halo, haloalkyl, —CN, —N(R)2, —N(R)C(O)R, —N(R)S(O)2R, or S(O)2R;
Rb is H, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, aryl, cycloalkyl, heteroaryl, heterocyclyl, —OR, —C(O)OR, —OC(O)R, halo, haloalkyl, —CN, —N(R)2, —N(R)C(O)R, —N(R)S(O)2R, or S(O)2R;
Rc is H, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, aryl, cycloalkyl, heteroaryl, heterocyclyl, —OR, —C(O)OR, —OC(O)R, halo, haloalkyl, —CN, —N(R)2, —N(R)C(O)R, —N(R)S(O)2R, or S(O)2R;
Rd is either absent, or when present, H, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, aryl, cycloalkyl, heteroaryl, heterocyclyl, —OR, —C(O)OR, —OC(O)R, halo, haloalkyl, —CN, —N(R)2, —N(R)C(O)R, —N(R)S(O)2R, or S(O)2R;
Re is either absent, or when present, H, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, aryl, cycloalkyl, heteroaryl, heterocyclyl, —OR, —C(O)OR, —OC(O)R, halo, haloalkyl, —CN, —N(R)2, —N(R)C(O)R, —N(R)S(O)2R, or S(O)2R;
Rg is either absent, or when present, H, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, aryl, cycloalkyl, heteroaryl, heterocyclyl, —OR, —C(O)OR, —OC(O)R, halo, haloalkyl, —CN, —N(R)2, —N(R)C(O)R, —N(R)S(O)2R, or S(O)2R;
Rw is either absent, or when present, H, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, aryl, cycloalkyl, heteroaryl, heterocyclyl, —OR, —C(O)OR, —OC(O)R, halo, haloalkyl, —CN, —N(R)2, —N(R)C(O)R, —N(R)S(O)2R, or S(O)2R;
Ry is H, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, aryl, cycloalkyl, heteroaryl, heterocyclyl, —OR, —C(O)OR, —OC(O)R, halo, haloalkyl, —CN, —N(R)2, —N(R)C(O)R, —N(R)S(O)2R, or S(O)2R;
Rz is H, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, aryl, cycloalkyl, heteroaryl, heterocyclyl, —OR, —C(O)OR, —OC(O)R, halo, haloalkyl, —CN, —N(R)2, —N(R)C(O)R, —N(R)S(O)2R, or S(O)2R;
n is independently 0, 1, 2, 3, 4 and 5;
R6 is C1-4 alkyl, phenyl, —C(O)R, —(CH2)nOR, —CN, F, Cl, Br, CF3, CF2H or CFH2, with the proviso that R is not H;
each R8 is independently H, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, aryl, cycloalkyl, heteroaryl, heterocyclyl, —OR, —C(O)OR, —OC(O)R, halo, haloalkyl, —CN, —N(R)2, —N(R)C(O)R, —N(R)S(O)2R, or S(O)2R;
R2, R3, R4, R5 and R7 are the same or different and either absent or, when present, independently H, C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, aryl, cycloalkyl, heteroaryl, heterocyclyl, —OR, —C(O)OR, —OC(O)R, halo, —CN, —N(R)2, —N(R)C(O)R, —N(R)S(O)2R, or S(O)2R;
wherein:
adjacent atoms on either the “a” ring or “b” ring may be joined together by single bonds to form a 9 atom carbocyclic or heterocyclic ring structure; or
atoms 1 and 2, 3 and 4, 5 and 6 and 8 and 9 on rings “a” ring and “b” ring may be joined together by double bonds to form an aromatic 9 atom carbocyclic or heterocyclic ring structure; or
atoms 1 and 2, 3 and 4, 6 and 7 and 8 and 9 on rings “a” ring and “b” ring may be joined together by double bonds to form an aromatic 9 atom carbocyclic or heterocyclic ring structure;
with the provisos that:
when Z is S or N, then A and C cannot both be N; and
when Z is N, then C and G cannot both be N.
|