| CPC C07D 265/26 (2013.01) | 20 Claims |
|
1. A process for the preparation of Salcaprozic Acid or a pharmaceutically acceptable salt thereof, comprising:
a. hydrolyzing a compound of formula II:
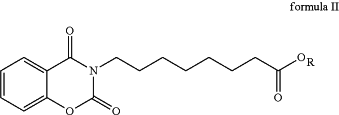 wherein, R is straight or branched chain alkyl
in the presence of benzotriazole, hydrazine, sodium borohydride or mixture thereof; and
b. isolating Salcaprozic Acid, which is free from colored impurity.
|