| CPC C07C 57/03 (2013.01) [C07D 303/38 (2013.01)] | 3 Claims |
|
1. An N-3 immunoresolvent compound having one of the formulae:
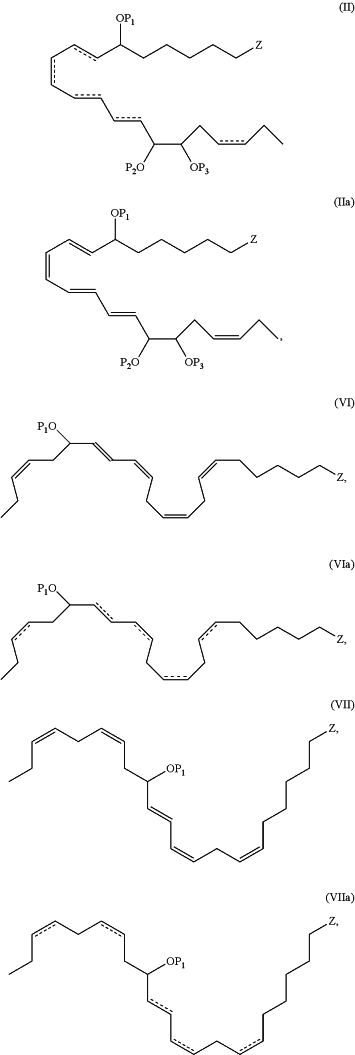 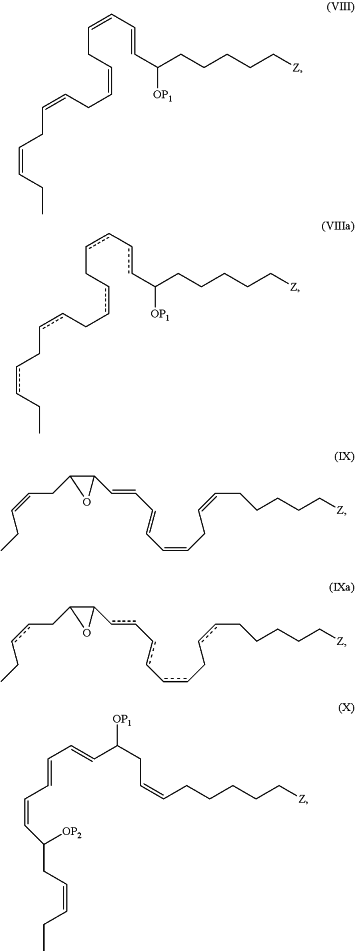 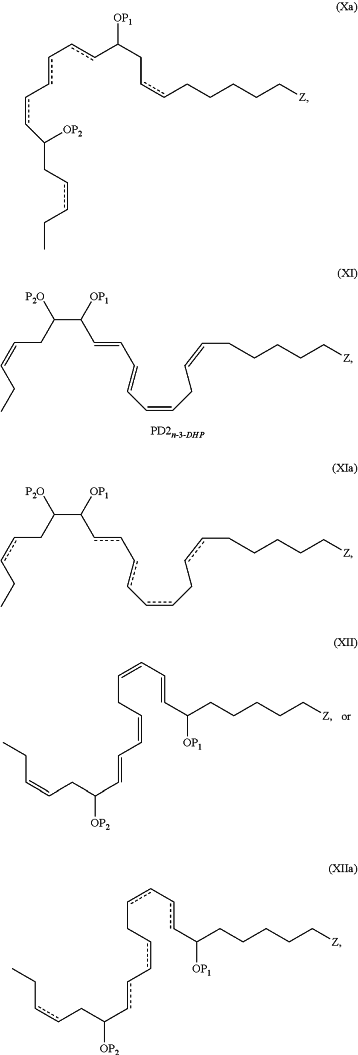 wherein each of P1, P2 and P3, when present, individually is a protecting group or a hydrogen atom;
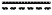 , when present, represents a double bond; , when present, represents a double bond;Z is —C(O)ORd, —C(O)NRcRc, —C(O)H, —C(NH)NRcRc, —C(S)H, —C(S)ORd, —C(S)NRcRc, or —CN;
each Ra, is independently selected from hydrogen, (C1-C6) alkyl, (C3-C8) cycloalkyl, cyclohexyl, (C4-C11) cycloalkylalkyl, (C5-C10) aryl, phenyl, (C6-C16) arylalkyl, benzyl, 2-6 membered heteroalkyl, 3-8 membered cycloheteroalkyl, morpholinyl, piperazinyl, homopiperazinyl, piperidinyl, 4-11 membered cycloheteroalkylalkyl, 5-10 membered heteroaryl or 6-16 membered heteroarylalkyl;
each Rc, is independently a protecting group or Ra, or, alternatively, each Rc is taken together with the nitrogen atom to which it is bonded to form a 5 to 8-membered cycloheteroalkyl or heteroaryl which may optionally include one or more of the same or different additional heteroatoms and which may optionally be substituted with one or more of the same or different Ra or suitable Rb groups;
each Rb is independently selected from ═O, —ORd, (C1-C3) haloalkyloxy, —OCF3, ═S, —SRd, ═NRd, ═NORd, —NRcRc, halogen, —CF3, —CN, —NC, —OCN, —SCN, —NO, —NO2, ═N2, —N3, —S(O)Rd, —S(O)2Rd, —S(O)2ORd, —S(O)NRcRc, —S(O)2NRcRc, —OS(O)Rd, —OS(O)2Rd, —OS(O)2ORd, —OS(O)2NRcRc, —C(O)Rd, —C(O)ORd, —C(O)NRcRc, —C(NH)NRcRc, —C(NRa)NRcRc, —C(NOH)Ra, —C(NOH)NRcRc, —OC(O)Rd, —OC(O)ORd, —OC(O)NRcRc, —OC(NH)NRcRc, —OC(NRa)NRcRc, —[NHC(O)]nRd, —[NRaC(O)]nRd, —[NHC(O)]nORd, —[NRaC(O)]nORd, —[NHC(O)]nNRcRc, —[NRaC(O)]nNRcRc, —[NHC(NH)]nNRcRc or —[NRaC(NRa)]nNRcRc;
each n, independently is an integer from 0 to 3; and
each Rd, independently is a protecting group or Ra;
or a pharmaceutically acceptable salt thereof, provided when Z is —C(O)ORd, then Rd for Z is not a hydrogen or a pharmaceutically acceptable salt thereof when P1, P2 and/or P3, when present, are all hydrogen atoms.
|