| CPC A61N 1/39622 (2017.08) [A61N 1/059 (2013.01); A61N 1/0597 (2013.01)] | 57 Claims |
|
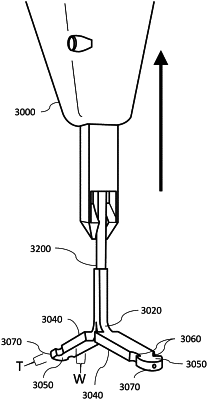
1. An electrical lead for implantation in a patient, the lead comprising:
a distal portion comprising one or more electrodes that are configured to generate therapeutic energy for biological tissue of the patient; and
a proximal portion coupled to the distal portion and configured to engage a controller, the controller configured to cause the one or more electrodes to generate the therapeutic energy,
wherein the distal portion is configured to split apart into sub-portions that travel in multiple directions during implantation into the patient, and
wherein the distal portion is wider than it is thick and the proximal portion is configured to be thinner than the distal portion in a manner that facilitates removal from a delivery system.
|