| CPC A61N 1/0548 (2013.01) [A61N 1/37518 (2017.08)] | 7 Claims |
|
1. A system comprising:
a lead having a proximal end and a distal end and defining an elongated lead body, wherein the lead is configured to be used for a trialing period when the lead is coupled to an external trial stimulator that is not to be implanted within a patient, and the same lead is configured to be used for chronic therapy when the lead is coupled to an implantable medical device after the trialing period;
one or more electrodes disposed on the lead;
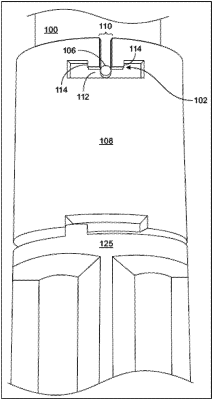
a fixation member disposed on the elongated lead body of the lead, wherein the fixation member is configured to secure the lead to tissue within the patient;
a trialing adaptor configured to receive the proximal end of the lead and configured to be removable from the lead when the trialing period is completed,
wherein the trialing adaptor is configured to couple the lead to the external trial stimulator,
wherein a first end of the trialing adaptor is configured to be within a body of the patient and a second end of the trialing adaptor is configured to exit from the body of the patient to provide a percutaneous connection from the lead to the external trial stimulator, and
wherein the proximal end of the lead includes one or more proximal connectors configured to couple to the implantable medical device upon implantation of the implantable medical device within the body of the patient after the trialing period; and
a sheath configured to enclose at least a portion of the lead and cover the fixation member, wherein the sheath is configured to remain in place over the at least a portion of the lead during the trialing period, wherein the sheath includes a connector pin disposed on the sheath that is configured to lock into the trialing adaptor, wherein the connector pin, when locked into the trialing adaptor, is configured to prevent movement of the sheath, and wherein the connector pin, when unlocked from the trialing adaptor, is configured to allow movement of the sheath.
|