| CPC A61M 16/0672 (2014.02) [A61M 2206/20 (2013.01)] | 18 Claims |
|
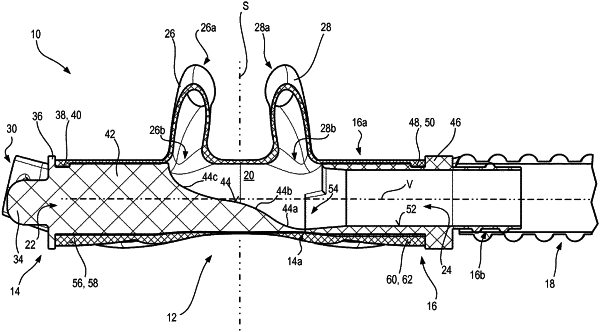
18. A nasal cannula therapy kit comprising:
a nasal cannula for nasally supplying a patient with therapeutic gas comprising a distribution conduit component having a distribution conduit that extends along a virtual distribution path passing centrally through it; the distribution path defining a longitudinal direction; the distribution conduit comprising two branch openings; a respective supply conduit for supplying the patient with the therapeutic gas from the distribution conduit branching off from each branch opening; the distribution conduit comprising, in addition to the branch openings, two coupling openings arranged at a distance from the branch openings; and
at least two plugs, wherein a closure opening of the two coupling openings is closable by one of the at least two plugs for a flow of gas through it; and a delivery opening of the two coupling openings being embodied for connection of a gas delivery line to the distribution conduit,
wherein each of a first plug and a second plug of the at least two plugs comprises a first flow-directing configuration having a first flow-directing surface; the first flow-directing surface being arranged, in a reference state in which either the first plug or the second plug is arranged on the nasal cannula so as to close off the closure opening, in the distribution conduit in a longitudinal region extending to at least one branch opening, and being canted with respect to the distribution path in such a way that it deflects the therapeutic gas, flowing in the distribution conduit in from the delivery opening along the distribution path, to at least one of the branch openings,
wherein a second plug of the at least two plugs comprises a second flow-directing configuration having a second flow-directing surface that is arranged at a distance along the distribution path from the first flow-directing configuration and that, in the reference state in which the second plug is arranged on the nasal cannula so as to close off the closure opening, the second flow-directing surface deflects the therapeutic gas, flowing in from the delivery opening along the distribution path, to the respective other branch opening,
wherein the first flow-directing configuration and the second flow-directing configuration differ in length, and/or
wherein the first flow-directing surface and the second flow-directing surface differ in conformation; and/or
the second flow-directing configuration extends over only a partial cross-section of the distribution conduit and, where the second flow-directing configuration extends it deflects the therapeutic gas flowing in along the distribution path into the respective other branch opening, and outside where the second flow-directing configuration extends, it constitutes, together with the distribution conduit, a flow-through opening for a flow of the therapeutic gas past the second flow-directing configuration to the first flow-directing surface, through which a portion of the therapeutic gas flowing in from the delivery opening can flow to the first flow-directing configuration.
|