| CPC A61K 38/179 (2013.01) [A61K 9/0019 (2013.01); A61K 39/39591 (2013.01); A61M 5/3129 (2013.01); C07K 16/22 (2013.01); A61K 45/06 (2013.01); A61K 2300/00 (2013.01); A61M 2005/3131 (2013.01); C07K 2317/24 (2013.01); C07K 2317/55 (2013.01); C07K 2317/76 (2013.01); C07K 2319/30 (2013.01)] | 20 Claims |
|
1. A pre-filled pharmaceutical package comprising a liquid formulation of a VEGF-antagonist, wherein the pre-filled pharmaceutical package comprises:
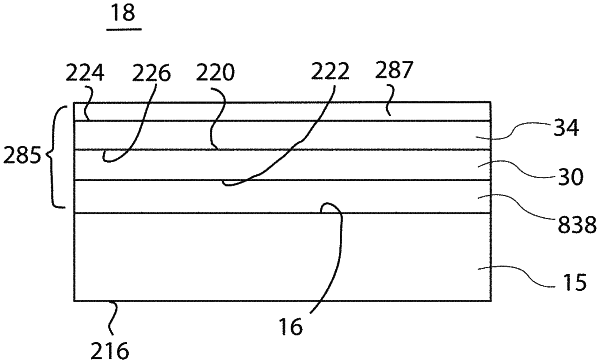
a wall comprising a cyclic olefin polymer, having an interior surface enclosing at least a portion of a lumen;
a tie coating or layer on the wall interior surface comprising SiOxCyHz, in which x is from about 0.5 to about 2.4 as measured by X-ray photoelectron spectroscopy (XPS), y is from about 0.6 to about 3 as measured by XPS, and z is from about 2 to about 9 as measured by at least one of Rutherford backscattering spectrometry (RBS) or hydrogen forward scattering (HFS);
a barrier coating or layer of SiOx, in which x is from about 1.5 to about 2.9 as measured by XPS, the barrier coating or layer positioned between the tie coating or layer and the lumen;
a pH protective coating or layer of SiOxCyHz, in which x is from about 0.5 to about 2.4 as measured by XPS, y is from about 0.6 to about 3 as measured by XPS, and z is from about 2 to about 9 as measured by at least one of RBS or HFS, positioned between the barrier coating or layer and the lumen;
a lubricity coating or layer positioned between the pH protective coating or layer and the lumen, wherein the lubricity coating or layer has the atomic proportions SiOxCyHz, in which x is from about 0.5 to about 2.4 as measured by XPS, y is from about 0.6 to about 3 as measured by XPS, and z is from about 2 to about 9 as measured by at least one of RBS or HFS;
a liquid formulation of a VEGF-antagonist in the lumen; and
a closure closing the lumen;
wherein the VEGF antagonist is Aflibercept.
|