| CPC A61K 31/4985 (2013.01) [A61K 9/14 (2013.01); A61K 9/2009 (2013.01); A61K 9/2013 (2013.01); A61K 9/2018 (2013.01); A61K 9/2059 (2013.01); A61K 9/2072 (2013.01); A61K 9/2077 (2013.01); A61K 9/2095 (2013.01); A61K 31/48 (2013.01)] | 22 Claims |
|
1. A dosage form comprising:
bromocriptine and one or more excipients;
wherein the dosage form provides for absorption of a substantial amount of bromocriptine through the gastric and/or intestinal mucosa when administered to a subject;
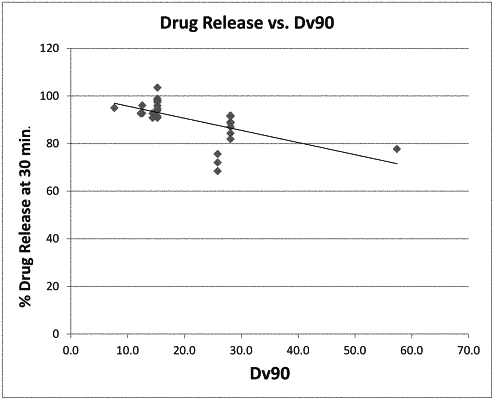
wherein the bromocriptine has a DV90 less than 15 μm and Dv10 of less than 2 μm; and
wherein the dosage form exhibits a pharmacokinetic profile wherein the time to maximum plasma concentration (Tmax) of bromocriptine is between about 30 and about 60 minutes following oral administration of the dosage form to the subject under fasting conditions or the Tmax of bromocriptine is between about 90 and about 120 minutes following oral administration of the dosage form to the subject under high fat fed conditions.
|