| CPC A61K 31/4196 (2013.01) [A61K 31/41 (2013.01); A61K 31/4439 (2013.01); A61K 31/506 (2013.01); A61P 35/00 (2018.01); A61P 35/02 (2018.01); C07D 249/12 (2013.01); C07D 401/06 (2013.01); C07D 401/10 (2013.01); C07D 403/06 (2013.01); C07D 403/10 (2013.01)] | 19 Claims |
|
1. A method for treating cancer comprising administering a therapeutically effective amount of a compound of Formula I and an anti-cancer agent to a mammal in need thereof:
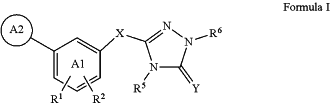 or a pharmaceutically acceptable salt thereof wherein:
A1 is phenyl or a 6-membered heteroaromatic ring having 1, 2 or 3 N in the heteroaromatic ring;
A2 is selected from A2a or A2b
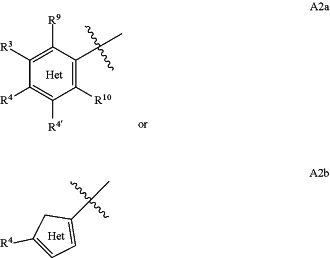 wherein A2a is phenyl or a 6 membered heteroaromatic ring having 1, 2 or 3 N in the heteroaromatic ring, and
A2b is a 5 membered heteroaromatic ring having 1, 2 or 3 heteroatoms independently selected from O, S and N;
X is selected from the group consisting of —(CH2)2—, —(CH2)3—, —(CH2)4, —(CH2)m—O—(CH2)n—, optionally mono- or di-substituted with halogen, wherein m and n are independently 0, 1, 2, 3 or 4, with the proviso that m+n is 2, 3, or 4;
Y is O;
R1 and R2 are each independently selected from the group consisting of:
(a) hydrogen,
(b) halogen,
(c) CN,
(d) CF3,
(e) —C1-6alkyl,
(f) —C1-6alkyl-C(═O)OH,
(g) —O—(R7),
(h) —S(═O)oR7,
(i) —N(R7)(R8),
(j) —N(R7)—C(═O)—(R8),
(k) —N(R7)—C(═O)—O—(R8),
(l) —N(R7)S(═O)2(R8),
(m) —C3-6cycloalkyl,
(n) —C(═O)(R7),
(o) aryl,
(p) heteroaryl,
(q) —OC(═O)N(R7)(R8),
(r) —S(═O)2N(R7)(R8),
(s) —C(═O)N(R7)(R8), and
(t) —C(R7)(R8)OH,
wherein the alkyl portion of choices (e) and (f), and the cycloalkyl portion of choice (m) are optionally substituted with halogen, and
wherein the aryl of choice (o) and the heteroaryl of choice (p) are optionally mono- or di-substituted with substituents selected from halogen, nitro, C1-6alkyl, C1-6alkoxy, halo C1-6alkyl, C3-6cycloalkyl, C3-6cycloalkoxy, —NH(C1-6alkyl), —NH(C3-6cycloalkyl), —N(C1-6alkyl)2, —N(C3-6cyc loalkyl)2, —S(═O)oC1-6alkyl, —S(═O)oC3-6cycloalkyl, and CN; each o is independently 0, 1, or 2;
R3 is selected from the group consisting of:
(a) hydrogen,
(b) halogen,
(c) CN,
(d) CF3,
(e) —C1-6alkyl,
(f) —C1-6alkyl-C(═O)OH,
(g) —O—(R7),
(h) —S(═O)oR7,
(i) —N(R7)(R8),
(j) —N(R7)—C(═O)—(R8),
(k) —N(R7)—C(═O)—O—(R8),
(l) —N(R7)S(═O)2(R8),
(m) —C3-6cycloalkyl,
(n) —C(═O)(R7),
(o) aryl,
(p) heteroaryl,
(q) —OC(═O)N(R7)(R8),
(r) —S(═O)2N(R7)(R8),
(s) —C(═O)N(R7)(R8),
(t) —C(R7)(R8)OH,
(u) —NHC(═O)—N(R7)(R8),
(v) —C3-6cycloalkyl-COOH,
(w) heterocycle, and
(x) —C1-6alkylC(═O)—N(R7)(R8),
wherein the alkyl portion of choices (e), (f) and (x), and the cycloalkyl portion of choices (m) and (v) are optionally substituted with halogen or hydroxyl, and
wherein the aryl of choice (o), the heteroaryl of choice (p), and the heterocycle of choice (w) are optionally mono- or di-substituted with substituents selected from halogen, nitro, C1-6 alkyl, C1-6alkoxy, halo C1-6alkyl, C3-6cycloalkyl, C3-6cycloalkoxy, —NH(C1-6alkyl), —NH(C3-6 cycloalkyl), —N(C1-6alkyl)2, —N(C3-6cycloalkyl)2, —S(═O)oC1-6alkyl, —S(═O)oC3-6cycloalkyl, hydroxyl and CN;
R4 and R4′ are each independently selected from the group consisting of:
(a) hydrogen,
(b) —N(R7)(R8),
(c) —N(R7)S(═O)2R8,
(d) —N(R7)—C(═O)R8,
(e) —N(R7)C(═O)OR8,
(f) —S(═O)oR7,
(g) —S(═O)2N(R7)(R8),
(h) —C(═O)R7,
(i) —C(═O)N(R7)(R8),
(j) —OC(═O)N(R7)(R8),
(k) —O—R7,
(l) —C(R7)(R8)OH,
(m) —C1-4alkyl-C(═O)NHS(═O)2R7,
(n) —C1-4alkyl-S(═O)2NHC(═O)R7,
(o) —C1-4alkyl-C(═O)—N(R7)(R8),
(p) —C1-4alkyl-N(R7)C(═O)(R8),
(q) —C1-4alkyl-N(R7) S(═O)2(R8),
(r) —C1-4alkyl-S(═O)2N(R7)(R8),
(s) —C1-4alkyl-N(R7)C(═O)O(R8)
(t) —C1-4alkyl-O—C(═O)N(R7)(R8)
(u) —C1-4alkyl-C(═O) (R7),
(v) —C1-4alkyl-C(R7)(R8) OH,
(w) —C1-4alkyl-O(R7),
(x) —C1-6alkyl-C(═O) OH,
(y) —C2-6alkenyl-C(═O)OH,
(z) —C3-6cycloalkyl-C(═O)OH,
(aa) —C3-6cycloalkyl-C(═O)NHS(═O)2R7,
(bb) —C3-6cycloalkyl-S(═O)2NHC(═O)R7,
(cc) —C3-6cycloalkyl-C(═O)—N(R7)(R8),
(dd) —C3-6cycloalkyl-N(R7) C(═O)(R8),
(ee) —C3-6cycloalkyl-N(R7)S(═O)2(R8),
(ff) —C3-6cycloalkyl-S(═O)2N(R7)(R8),
(gg) —C3-6cycloalkyl-N(R7)C(═O)O(R8),
(hh) —C3-6cycloalkyl-O—C(═O)N(R7)(R8),
(ii) —C3-6cycloalkyl-C(═O)(R7),
(jj) —C3-6cycloalkyl-C(R7)(R8)OH,
(kk) —C3-6cycloalkyl-O(R7),
(ll) —C(═O)OH,
(mm) aryl,
(nn) heteroaryl,
(oo) —C(═O)N(R7)S(═O)2(R8),
(pp) —S(═O)2N(R7)C(═O)(R8),
(qq) —NHS(═O)2N(R7)(R8),
(rr) —NHC(═O)N(R7)(R8),
(ss) —CH(OH)—C(═O)—N(R7)(R8),
(tt) —C(═O)—C(═O)—N(R7)(R8),
(uu) —C3-6cycloalkyl,
(w) —CF3,
(ww) —C1-6alkyl N(R7)(R8),
(xx) -heterocycle,
(yy) —C1-6alkyl,
(zz) halogen, and
(aaa) —O—C1-6alkyl-N(R7)(R8),
wherein the alkyl portion of choices (m), (n), (o), (p), q), (r), (s), (t), (u), (v), (w), (x), (ww), (yy) and (aaa), the alkenyl portion of choice (y), and the cycloalkyl portion of choices (aa), (bb), (cc), (dd), (cc), (gg), (hh), (ii), (jj), (kk) and (uu), are optionally mono- or di-substituted with halogen, CN, aryl, C1-6alkyl, halo C1-6alkyl, C3-6cycloalkyl, C1-6alkoxy, or C3-6cycloalkoxy, and
wherein the aryl of choice (mm), the heteroaryl of choice (nn), and the heterocycle of choice (xx) are optionally mono- or di-substituted with substituents selected from halogen, nitro, C1-6alkyl, C1-6alkoxy, halo C1-6alkyl, C3-6cycloalkyl, C3-6cycloalkoxy, —NH(C1-6alkyl), —NH(C3-6 cycloalkyl), —N(C1-6alkyl)2, —(C3-6cycloalkyl)2, —S(═O)oC3-6cycloalkyl, hydroxyl and CN, or
wherein R3 and R4 or R4 and R4′ are joined together to form a 5- or 6-membered heterocyclic ring, said ring having one heteroatom selected from O and N, wherein said ring is optionally substituted with —C(═O)OH, or —C1-6alkyl-C(═O)OH, with the proviso that at least one of R3, R4 and R4′ is other than hydrogen;
R5 is selected from the group consisting of:
(a) hydrogen,
(b) —C1-6alkyl,
(c) —C1-4 alkyl(R7),
(d) aryl,
(e) heteroaryl,
(f) —C3-6cycloalkyl,
(g) —C3-6cycloalkyl(R7),
(h) —C3-6cycloalkyl-O(R7),
(i) —C1-4 alkyl-C3-6cycloalkyl,
(j) C1-6alkoxy, and
(k) C3-6cycloalkoxy,
wherein the alkyl portion of choices (h), (c), (i) and (j), the cycloalkyl portion of choices (f), (g), (h), (i) and (k) are optionally substituted with halogen or C1-4alkyl, and
wherein the aryl of choice (d) and the heteroaryl of choice (e), are optionally mono- or di-substituted with substituents selected from halogen, nitro, C1-6alkyl, CF3, C1-6alkoxy, halo C1-6alkyl, aryl, heteroaryl, C3-6cycloalkyl, C3-6cycloalkoxy, and CN;
R6 is selected from the group consisting of:
(a) hydrogen,
(b) —C1-6alkyl,
(c) —C1-6alkylaryl,
(d) —C1-6alkylheteroaryl,
(e) —S(═O)oC1-6alkyl(R7),
(f) —C(═O)C1-6alkyl(R7),
(g) —C3-6cycloalkyl,
(h) aryl,
(i) heteroaryl,
(j) —C(═O)C3-6cycloalkyl(R7),
(k) —S(═O)oC3-6cycloalkyl(R7), and
(l) —C1-6alkyl(R7),
wherein the alkyl portion of choices (b), (c), (d), (e), (f), and (l) and the cycloalkyl portion of choices (g), (j), and (k), are optionally substituted with halogen or C1-4alkyl, and
wherein the aryl portion of choices (c) and (h), and the heteroaryl portion of choices (d) and (i), are optionally mono- or di-substituted with substituents selected from halogen, nitro, —CF3, C1-6alkyl, C1-6alkoxy, halo C1-6alkyl, C3-6cycloalkyl, C3-6cycloalkoxy, aryl, heteroaryl, heterocycle optionally substituted with halogen, —NH(C1-6alkyl), —NH(C3-6cycloalkyl) —N(C1-6alkyl)2, —N(C3-6cycloalkyl)2, —S(═O)oC1-6alkyl, S(═O)oC3-6cycloalkyl, and CN;
R7 and R8 are each independently selected from the following:
(a) hydrogen,
(b) —C1-6alkyl,
(c) —C3-6cycloalkyl,
(d) -aryl,
(e) -heteroaryl,
(f) —C1-6alkylaryl,
(g) —C1-6alkylheteroaryl,
(h) —C(═O)C1-6alkyl,
(i) —S(═O)o-aryl,
(j) —C1-6 alkyl-C3-6cycloalkyl, and
(k) CF3,
wherein the alkyl of choices (h), (f), (g), (h), and (j), and the cycloalkyl of choices (c) and (j), are each optionally mono-, di- or tri-substituted with halogen, and
wherein the aryl portion of choices (d), (f) and (i), and the heteroaryl portion of choices (e) and (g), are each optionally mono- or di-substituted with substituents selected from halogen, —C(═O)OH, —CF3, —NHC(═O)CH3, nitro, C1-6alkyl, C1-6alkoxy, halo C1-6alkyl, C3-6cycloalkyl, C3-6cycloalkoxy, —NH(C1-3alkyl), —NH(C3-6cycloalkyl), —N(C1-3alkyl)2, —N(C3-6cycloalkyl)2, —S(═O)oC1-4alkyl, S(═O)oC3-6cycloalkyl, aryl, heteroaryl, hydroxyl, and CN;
R9 and R10 are each independently selected from the following
(a) hydrogen,
(b) —C1-6alkyl,
(c) —C3-6cycloalkyl,
(d) halogen,
(e) —OC3-6cycloalkyl,
(f) CF3, and
(g) C1-6alkoxy,
wherein the alkyl portion of choice (b) and the cycloalkyl portion of choices and (e), are each optionally mono-, di- or tri-substituted with halogen;
wherein the cancer is selected from the group consisting of prostate cancer, breast cancer, ovarian cancer, liver cancer, kidney cancer, colon cancer, pancreatic caner, human chronic lymphocytic leukemia, and melanoma.
|