| CPC C07D 471/04 (2013.01) [C07D 519/00 (2013.01); C12Q 1/66 (2013.01); G01N 33/52 (2013.01); G01N 33/533 (2013.01); G01N 33/542 (2013.01); G01N 2458/15 (2013.01)] | 9 Claims |
|
1. A compound of formula (I)
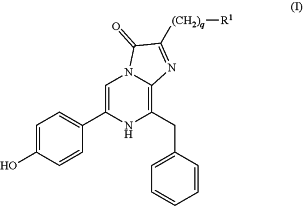 or a tautomer or a salt thereof, wherein:
R1 is optionally substituted aryl, optionally substituted heteroaryl, optionally substituted heterocycle, or optionally substituted cycloalkyl, wherein R1 is substituted with at least one group that is -Q-L-Z;
Q is —O—, —NRQ—, —NRQ—CO—, —CO—NRQ—, —O—CO—NRQ—, or —NRQ—CO—O—;
L is —(CR1aR1b)m—, —(CR1xR1y—CR1xR1y—O)t1—(CR1xR1y)t2—Q1—, —(CR1xR1y)t2-A-(CR1xR1y—CR1xR1y—O)t1-Q1-, wherein each Q1 is independently a bond, —O—, or —NRQ—, and A is a bond, —O—, —NRQ—, —NRQ—CO—, —CO—NRQ—, —O—CO—NRQ—, or —NRQ—CO—O—;
Z is —COOR2, —SO2—OR3, —PO(OR4)(OR5), halo, azide, C2-C10 alkynyl, a biotin moiety, —NR6R7, or —NR8—CO—R9;
R2, R3, R4, R5, R8, R9, R10, R11, and R12 at each occurrence are independently hydrogen, optionally substituted C1-C8 alkyl, optionally substituted aryl, optionally substituted cycloalkyl, optionally substituted heteroaryl, optionally substituted heterocycle, optionally substituted heteroalkyl, or an energy acceptor;
R6 and R7 at each occurrence are independently hydrogen, optionally substituted C1-C8 alkyl, optionally substituted aryl, optionally substituted cycloalkyl, optionally substituted heteroaryl, optionally substituted heterocycle, optionally substituted heteroalkyl, or an energy acceptor; or R6 and R7, together with the nitrogen atom to which they are attached, together form an optionally substituted ring;
R1a, R1b, RQ, RQ1, R1x, and R1y at each occurrence are independently hydrogen, C1-C4 alkyl, or C1-C4 haloalkyl;
q is 0, 1, or 2;
m at each occurrence is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, or 12;
t1 at each occurrence is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10; and
t2 is an integer from 0-5;
wherein, when a group is optionally substituted, it is either unsubstituted or substituted with one or more substituents independently selected from halogen, ═O, ═S, cyano, nitro, alkyl, alkenyl, alkynyl, haloalkyl, haloalkoxy, heteroalkyl, cycloalkyl, cycloalkenyl, aryl, heteroaryl, heterocycle, cycloalkylalkyl, heteroarylalkyl, arylalkyl, hydroxy, hydroxyalkyl, alkoxy, alkoxyalkyl, aryloxy, benzyloxy, amino, alkylamino, acylamino, aminoalkyl, arylamino, sulfonylamino, sulfinylamino, sulfonyl, alkylsulfonyl, arylsulfonyl, aminosulfonyl, sulfinyl, —COOH, amide, carbamate, and acyl; and
wherein the compound is not 4-[4-[[8-benzyl-6-(4-hydroxyphenyl)-3-oxo-7H-imidazo[1,2-a]pyrazin-2-yl]methyl]phenoxy]butanoic acid.
|