| CPC C07D 417/14 (2013.01) [C07D 277/52 (2013.01); C07D 417/12 (2013.01)] | 19 Claims |
|
1. A compound of formula (I):
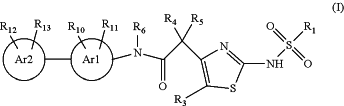 wherein
R1 is C1-5alkyl, C0-2alkyleneC3-5cycloalkyl which cycloalkyl is optionally substituted by CH3, C1-3alkyleneOC1-2alkyl, or CF3;
R3 is H, halo, CH3, OC1-2alkyl or CF3;
or R3 together with R5 forms a 5- or 6-membered cycloalkyl or 5 or 6 membered oxygen-containing heterocycloalkyl;
R4 and R5 are each independently H, halo, C1-6alkyl, C0-2alkyleneC3-6cycloalkyl, C0-2alkyleneC3-6heterocycloalkyl, OC1-6alkyl, OC0-2alkyleneC3-6cycloalkyl, C1-3alkyleneOC1-3alkyl, C1-6alkylOH, C1-6haloalkyl, OC1-6haloalkyl or NR21R22,
or R4 is H and R5 together with R3 form a 5- or 6-membered cycloalkyl or 5 or 6 membered oxygen-containing heterocycloalkyl,
or R4 and R5 together with the carbon atom to which they are attached form a C3-6cycloalkyl or C3-6heterocycloalkyl,
or R4 is H and R5 and R6 are a C2-3alkylene chain forming a 5- or 6-membered ring;
or R4 is O and R5 is absent;
R6 is H or C1-3alkyl,
or R6 together with Rn when in the ortho-position to the amide are a C2alkylene chain forming a 5-membered ring,
or R5 and R6 are a C2-3alkylene chain forming a 5- or 6-membered ring and R4 is H;
Ar1 is 6-membered aryl or 6-membered heteroaryl;
Ar2 is a 6-membered aryl or 6-membered heteroaryl and is attached to Ar1 in the para position relative to the amide;
R10 is H, halo, C1-3alkyl, OC1-2alkyl, C1-2haloalkyl, OC1-2haloalkyl or CN;
R11 is H, F, Cl, CH3, ethyl, OCH3, CF3, OCF3 or CN,
or R11, when in the ortho-position to the amide, together with R6 are a C2alkylene chain forming a 5-membered ring;
R12 is attached to Ar2 in the ortho or meta position relative to Ar1 and R12 is H, halo, C1-4alkyl, C2-4alkynyl, C0-2alkyleneC3-5cycloalkyl, OC1-4alkyl, OC0-2alkyleneC3-5cycloalkyl, OCH2CH2N(CH3)2, OH, C1-4alkylOH, CN, C1-3alkyleneOC1-3alkyl, C1-4haloalkyl, OC1-4haloalkyl, C(═O)C1-2alkyl, NR23R24, SO2C1-4alkyl, SOC1-4alkyl, SC1-4alkyl, SH, C(O)N(CH3)2, NHC(O)C1-3alkyl, C3-6heterocycloalkyl comprising one nitrogen located at the point of attachment to Ar2, or R12 together with a nitrogen atom to which it is attached forms an N-oxide (N+—O−);
R13 is H, halo, CH3 or OCH3;
R21 is H, C1-5alkyl, C(O)C1-5alkyl, C(O)OC1-5alkyl;
R22 is H or CH3;
R23 is H or C1-2alkyl; and
R24 is H or C1-2alkyl;
or a salt, solvate, or salt and solvate thereof.
|