| CPC C07D 403/12 (2013.01) [A61K 31/517 (2013.01); A61K 45/06 (2013.01); A61P 11/00 (2018.01); C07D 239/84 (2013.01); C07D 401/14 (2013.01); C07D 403/14 (2013.01); C07D 417/14 (2013.01)] | 16 Claims |
|
1. A compound shown in formula I, a pharmaceutically acceptable salt thereof, a tautomer thereof, or a stereoisomer thereof, having a structure shown as below:
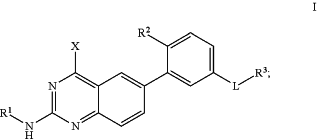 wherein, X is hydrogen;
R3 is unsubstituted or R3-1 substituted C6-10 aryl, unsubstituted or R3-2 substituted 5 to 10 membered heteroaryl with 1 to 4 heteroatoms selected from one or more of N, O and S, or unsubstituted or R3-3 substituted C6-10 aryl-fused C3-8 cycloalkyl;
R3-1 and R3-2 are independently hydroxyl, unsubstituted or R3-1-1 substituted C1-6 alkyl, C1-6 alkoxy, or unsubstituted or R3-1-4 substituted C6-10 aryl;
R3-3 is independently hydrogen or C1-6 alkyl substituted with one or more halogens;
R3-1-1 and R3-1-4 are independently cyano, halogen, or unsubstituted or R3-1-1-1 substituted 3 to 10 membered heterocycloalkyl with 1 to 3 heteroatoms selected from one or more of N, O and S;
R3-1-1-1 is C1-4 alkyl;
R2 is methyl, ethyl, isopropyl or cyclopropyl;
when R2 is methyl or ethyl, then L is —NH—CO—NH—, —CHR4-1—CO—NH—, —CHR4-2—NH—CO—, —NH—CO—CHR4-3—, —CO—NH—CHR4-4—, —CHR4-5—NH—CO—NH—, —NH—CO—NH—CHR4-6—, —CO—NH—CR4-7R4-8—
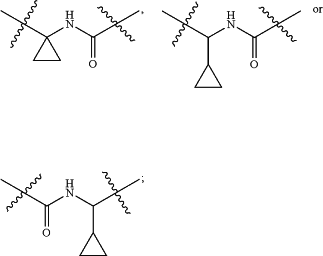 when R2 is methyl or ethyl, then R1 is unsubstituted or R1-1 substituted C6-10 aryl, or unsubstituted or R1-2 substituted 5 to 10 membered heteroaryl with 1 to 4 heteroatoms selected from one or more of N, O and S;
when R2 is isopropyl or cyclopropyl, then L is —CO—NH—, —NH—CO—, —NH—CO—NH—, —CHR4-1—CO—NH—, —CHR4-2—NH—CO—, —NH—CO—CHR4-3—, —CO—NH—CHR4-4—, —CHR4-5—NH—CO—NH—, —NH—CO—NH—CHR4-6—, —CO—NH—CR4-7R4-8—,
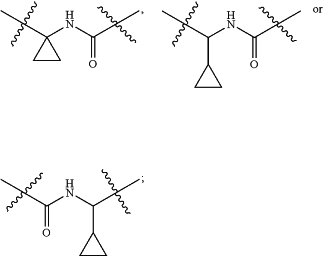 when R2 is isopropyl or cyclopropyl, then R1 is unsubstituted or R1-1 substituted C6-10 aryl, or unsubstituted or R1-2 substituted 5 to 10 membered heteroaryl with 1 to 3 heteroatoms selected from one or more of N, O and S;
the left end of L is connected to
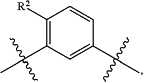 and the right end is connected to R3;
R4-1, R4-2, R4-3, R4-4, R4-5, R4-6, R4-7 and R4-8 are independently hydrogen, C1-4 alkyl, or R4-7 and R4-8 together with the carbon atom therebetween form C3-6 cycloalkyl;
R1-1 and R1-2 are independently unsubstituted or R1-1-1 substituted C1-6 alkyl, C1-6 alkoxy, unsubstituted or R1-1-2 substituted C3-10 cycloalkyl, unsubstituted or R1-1-3 substituted 3 to 10 membered heterocycloalkyl with 1 to 3 heteroatoms selected from one or more of N, O and S, or NR1-1-4R1-1-5;
R1-1-1, R1-1-2, R1-1-3, R1-1-4 and R1-1-5 are independently hydroxyl or C1-6 alkyl.
|