| CPC C07D 221/20 (2013.01) [C07D 211/22 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 405/12 (2013.01); C07D 417/12 (2013.01); C07D 417/14 (2013.01)] | 8 Claims |
|
1. A compound having the structural Formula (I):
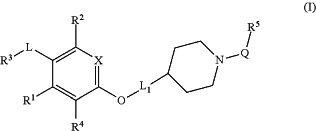 or a pharmaceutically acceptable salt thereof, wherein:
X is CH or N;
R1 is selected from H, methyl, and halogen;
R2 is selected from H, Cl, cyano, cyclopropyl, —CH3, and —OCH3;
R4 is selected from H, Cl, and methyl;
-L- is a divalent moiety C(O)—;
R3 is —N(RN1)(RN2), wherein RN1 and RN2 are taken together with the nitrogen atom to which they are shown attached to form a 4-, 5-, or 6-membered fully saturated heterocyclic ring wherein said heterocyclic ring having 1, 2, or 3 ring heteroatoms selected from N, N-oxide, O, S, and S-oxide,
wherein said heterocyclic ring is unsubstituted or substituted with 1, 2, or 3 substituents independently selected from halogen, —OH, oxo, CN, —(C1-C6)alkyl, amino-substituted —(C1-C6)alkyl, —O—(C1-C6)alkyl, —(C1-C6)alkyl-OH, —(C1-C6)haloalkyl, —C(O)O—(C1-C6)alkyl, cyclopropyl, spirocyclopropyl, —CH2—NHC(O)O—(C1-C6)alkyl, —CH2—N(CH3)C(O)O—(C1-C6)alkyl, phenyl, benzyl, —NHC(O)-phenyl, heteroaryl, —(C1-C4)alkylheteroaryl, and heterocycloalkyl, wherein 1, 2, or 3 groups of said amino substituent on the amino-substituted —(C1-C6)alkyl is independently selected from —NH2, —N(C1-C4alkyl)2, and —NH(C1-C4alkyl);
-L1- is a divalent moiety selected from:
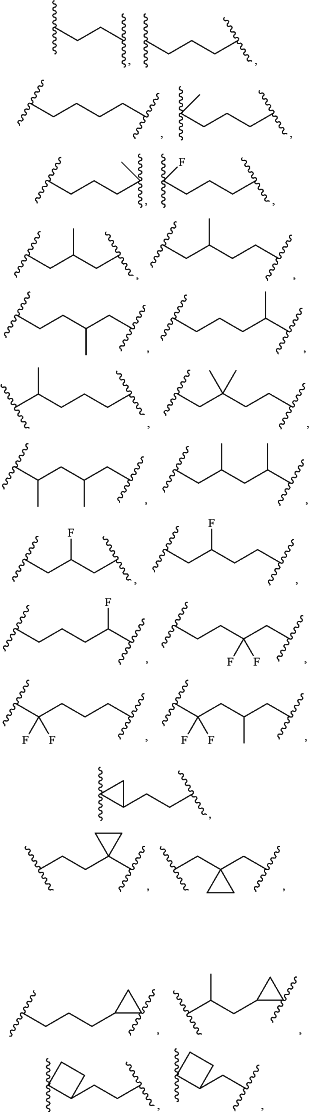 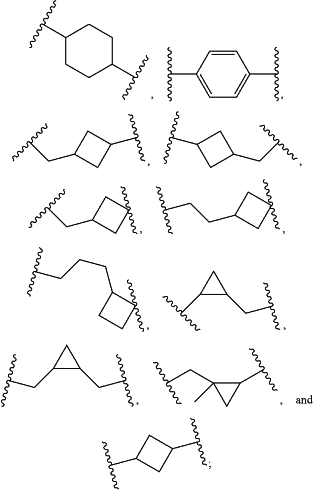 Q is a bond or a divalent moiety selected from —C(O)—, and —S(O)2—; and
R5 is selected from:
1) —C(R5A)(R5B)(R5C), wherein:
R5A is OH;
R5B is —(C1-C3)fluoroalkyl; and
R5C is selected from NH2, NHCH3, —(C1-C6)alkyl, —(C1-C4)fluoroalkyl, thiadiazolyl, thienyl, thiazolyl, ethenyl, ethynyl, phenyl, cyclopropyl, and cyclobutyl; wherein said phenyl is substituted with from 1-3 groups independently selected from halogen —(C1-C6)alkyl, and —(C1-C6)alkoxy; wherein said cyclopropyl or cyclobutyl is optionally substituted with —(C1-C6)alkyl;
2)
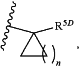 wherein n is an integer from 1 to 4;
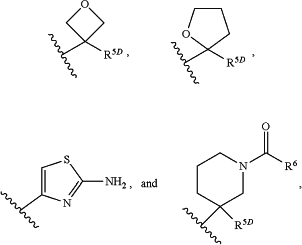 wherein R5D is selected from H, —(C1-C6)alkyl, —(C1-C6)haloalkyl, pyrimidinyl, phenyl, and said phenyl substituted with from 1 to 3 groups independently selected from OH, halogen, —(C1-C6)alkyl, and —O—(C1-C6)alkyl; and R6 is H or CH3, —O—(C1-C6)alkyl; and
3) unsubstituted phenyl or phenyl substituted with 1, 2, or 3 groups independently selected from halogen, CN, —(C1-C6)alkyl, —(C1-C6)haloalkyl, and pyrrolidinyl.
|