| CPC A61M 5/3287 (2013.01) [A61B 17/24 (2013.01); A61B 17/3468 (2013.01); A61K 9/0024 (2013.01); A61L 31/16 (2013.01); A61M 5/3286 (2013.01); A61M 2205/04 (2013.01); A61M 2210/0681 (2013.01)] | 24 Claims |
|
1. A method for sinus treatment, comprising:
grasping a proximal grip portion of a surgical device outside of a patient;
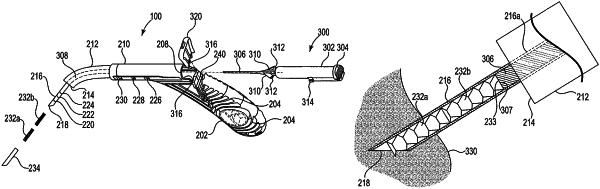
manipulating the proximal grip portion to pierce mucosal turbinate tissue of the patient with a distal hollow sharp needle portion of the surgical device and submucosally insert the distal hollow sharp needle portion into the mucosal turbinate tissue; and
activating an actuator disposed within the surgical device to deliver one or more biodegradable, drug-eluting solid implants from the distal hollow sharp needle portion into the mucosal turbinate tissue and submucosally bury at least one implant withdrawal discouraging, mucosal tissue-engaging surface feature along a length of each of the one or more implants within the mucosal turbinate tissue.
|