| CPC A61K 39/12 (2013.01) [A61K 9/0021 (2013.01); A61K 39/0241 (2013.01); A61K 2039/521 (2013.01); A61K 2039/5254 (2013.01); A61K 2039/54 (2013.01); A61K 2039/70 (2013.01); C12N 2750/10034 (2013.01); C12N 2770/10034 (2013.01)] | 9 Claims |
|
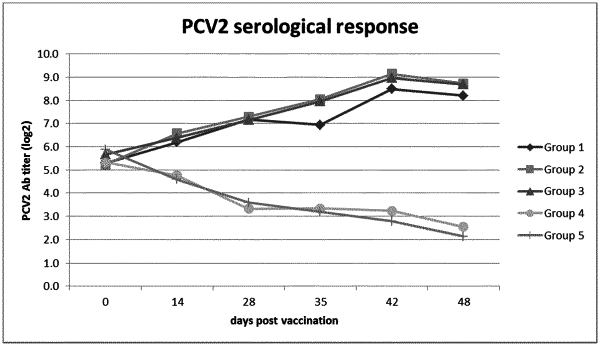
1. A single-dose vaccine comprising in combination a non-replicating immunogen of porcine circovirus type 2 (PCV2) and a live attenuated porcine reproductive and respiratory syndrome (PRRS) virus, wherein the vaccine is administered intradermally for use in prophylactically treating an animal against an infection of PCV2, PRRS virus, or both PCV2 and PRRS virus.
|