| CPC A61F 9/0017 (2013.01) [A61J 1/1468 (2015.05); A61K 9/0048 (2013.01); A61K 39/3955 (2013.01); A61L 31/08 (2013.01); A61L 31/088 (2013.01); A61L 31/14 (2013.01); A61M 5/3129 (2013.01); A61M 5/31505 (2013.01); A61M 5/3202 (2013.01); A61M 5/5086 (2013.01); C23C 16/045 (2013.01); C23C 16/401 (2013.01); C23C 16/505 (2013.01); A61L 2400/10 (2013.01); A61L 2420/08 (2013.01); A61M 5/002 (2013.01); A61M 2005/3131 (2013.01); A61M 2205/0222 (2013.01); A61M 2207/10 (2013.01)] | 32 Claims |
|
1. An ophthalmic drug in a pre-filled pharmaceutical package comprising:
a syringe barrel having a front dispensing opening and a back opening, the syringe barrel comprising a thermoplastic wall having an interior surface enclosing at least a portion of a lumen, an exterior surface, and a coating set on at least one of the interior surface and the exterior surface of the wall, the coating set comprising:
a tie coating or layer on the interior surface or the exterior surface comprising SiOxCyHz in which x is from about 0.5 to about 2.4 as measured by X-ray photoelectron spectroscopy (XPS), y is from about 0.6 to about 3 as measured by XPS, and z is from about 2 to about 9 as measured by at least one of Rutherford backscattering spectrometry (RBS) or hydrogen forward scattering (HFS), the tie coating or layer having a facing surface facing toward the wall, the tie coating or layer also having an opposed surface facing away from the wall;
a barrier coating or layer of SiOx, in which x is from about 1.5 to about 2.9 as measured by XPS, the barrier coating or layer having a facing surface facing toward the opposed surface of the tie coating or layer and an opposed surface facing away from the tie coating or layer;
a pH protective coating or layer of SiOxCyHz, in which x is from about 0.5 to about 2.4 as measured by XPS, y is from about 0.6 to about 3 as measured by XPS, and z is from about 2 to about 9 as measured by at least one of RBS or HFS, the pH protective coating or layer having a facing surface facing toward the opposed surface of the barrier layer and an opposed surface facing away from the barrier layer;
in the lumen, a liquid formulation of an ophthalmic drug suitable for intravitreal injection; and
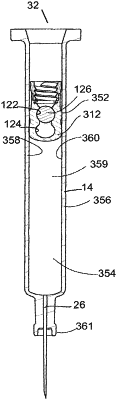
a closure seated in the lumen having a front face facing the liquid formulation, the closure being an axially stretchable plunger in the syringe barrel and axially slidable toward the front dispensing opening, the plunger comprising: an elastomeric sleeve having a sidewall and a front face facing the front dispensing opening, the sidewall comprising a stretch zone that is adapted to undergo axial elongation to convert the plunger from a storage mode to a dispensing mode, wherein the elongation reduces an outer profile of at least a portion of the sidewall, thus reducing the plunger to a constricted state;
wherein the plunger further comprises a liquid sealing section when the plunger is in the constricted state, and wherein the liquid sealing section comprises a non-stretch zone of the sidewall of the elastomeric sleeve; and
wherein the syringe barrel and closure are free of silicone oil and baked-on silicone.
|