| CPC H04L 9/0866 (2013.01) [H04L 63/0428 (2013.01); H04L 63/06 (2013.01); H04L 63/166 (2013.01); H04L 63/168 (2013.01); H04L 2209/88 (2013.01)] | 20 Claims |
|
1. A cloud system for storing a test result, comprising:
a computer-readable memory storing executable instructions; and
one or more hardware-based processors programmed by the executable instructions to perform a method comprising:
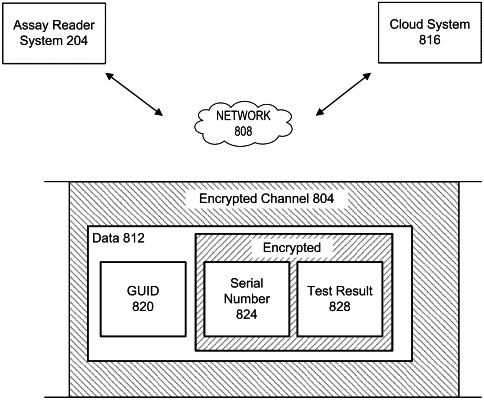
receiving a global unique identifier and an encrypted data block from a diagnostic test device via a communication channel, wherein the encrypted data block comprises a received serial number and a test result;
obtaining a stored serial number and an encryption key associated with the global unique identifier;
authenticating the diagnostic test device by decrypting the encrypted data block using the encryption key to obtain the received serial number and the test result and determining if the received serial number and the stored serial number are identical; and
if the received serial number and the stored serial number are the same, then storing the test result in a storage device.
|