| CPC C07D 215/233 (2013.01) [A61P 33/06 (2018.01); C07D 401/12 (2013.01); C07D 405/12 (2013.01); C07D 471/04 (2013.01); C07D 487/04 (2013.01)] | 17 Claims |
|
1. A compound of the structure of
(a) Formula (I):
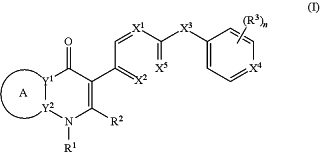 or a stereoisomer thereof, or a pharmaceutically acceptable salt thereof,
wherein
ring A combines with Y1 and Y2 to form a C3-7cycloalkenyl or heteroaryl ring,
wherein the C3-7cycloalkenyl or heteroaryl is optionally substituted by halogen, C1-3alkyl, C1-3alkoxy, C1-3haloalkyl, —O—C1-3haloalkyl, —S—C1-3haloalkyl, —C(O)OR, cyano or phenyl;
Y1 is N;
Y2 is C or N;
X1 is C(Rx1) or N,
wherein Rx1 is hydrogen, halogen, C1-3alkyl, C1-3alkoxy or C1-3haloalkyl;
X2 is C(Rx2) or N,
wherein Rx2 is hydrogen, halogen, C1-3alkyl, C1-3alkoxy or C1-3haloalkyl;
X3 is O, N(R), S or C1-3alkyl;
X4 is C or N;
X5 is C or N;
R1 is hydrogen or C1-3alkyl;
R2 is hydrogen, C1-3alkyl, C1-3haloalkyl, —CH2OH, —CH2OR or —C(O)OR;
n is 0, 1, 2, 3 or 4;
each R3 is independently halogen, C1-3alkyl, C1-3alkoxy, C1-3haloalkyl, —O—C1-3haloalkyl, —S—C1-3haloalkyl, —C(O)OR or SF5;
or two R3 groups, together with the carbons to which they are attached, form a 1,3-dioxolane; and
each R is independently hydrogen or C1-3alkyl; or
(b) Formula (I-p):
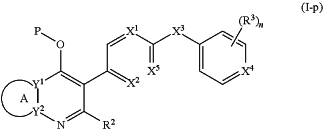 or a stereoisomer thereof, or a pharmaceutically acceptable salt thereof,
wherein
ring A combines with Y1 and Y2 to form a C3-7cycloalkenyl or heteroaryl ring, wherein the C3-7cycloalkenyl or heteroaryl is optionally substituted by halogen, C1-3alkyl, C1-3alkoxy, C1-3haloalkyl, —O—C1-3haloalkyl, —S—C1-3haloalkyl, —C(O)OR, cyano or phenyl;
Y1 is N;
Y2 is C or N;
X1 is C(Rx1) or N,
wherein Rx1 is hydrogen, halogen, C1-3alkyl, C1-3alkoxy or C1-3haloalkyl;
X2 is C(Rx2) or N,
wherein Rx2 is hydrogen, halogen, C1-3alkyl, C1-3alkoxy or C1-3haloalkyl;
X3 is O, N(R), S or C1-3alkyl;
X5 is C or N;
P is —C(O)OR′, —C(O)R′, —C(O)NR′2, wherein R′ is hydrogen, C1-3alkyl or —CH2OR;
R2 is hydrogen, C1-3alkyl, C1-3haloalkyl, —CH2OH, —CH2OR, —C(O)OR or —CH2OP;
P is —C(O)OR′, —C(O)R′, —C(O)NR′2 or —OP(O)(OR′)OR′, wherein each R′ is independently hydrogen or C1-3alkyl;
n is 0, 1, 2, 3 or 4;
each R3 is independently halogen, C1-3alkyl, C1-3alkoxy, C1-3haloalkyl, —O—C1-3haloalkyl, —S—C1-3haloalkyl, —C(O)OR or SF5;
or two R3 groups, together with the carbons to which they are attached, form a 1,3-dioxolane; and
each R is independently hydrogen or C1-3alkyl.
|
|
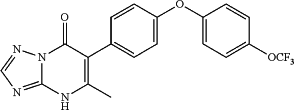
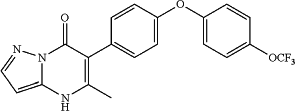
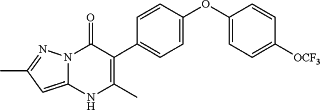
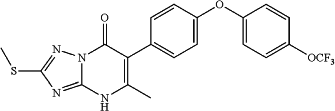
14. A compound that is:
| |||||||||||||||||||||||||||
5-methyl-6-(4-(4-(trifluoromethoxy)phenoxy)phenyl)-[1,2,4]triazolo[1,5-a]pyrimidin-7(4H)-one;
5-methyl-6-(4-(4-(trifluoromethoxy)phenoxy)phenyl)pyrazolo[1,5-a]pyrimidin-7(4H)-one;
2,5-dimethyl-6-(4-(4-(trifluoromethoxy)phenoxy)phenyl)pyrazolo[1,5-a]pyrimidin-7(4H)-one;
5-methyl-2-(methylthio)-6-(4-(4-(trifluoromethoxy)phenoxy)phenyl)-[1,2,4]triazolo [1,5-a]pyrimidin-7(4H)-one;
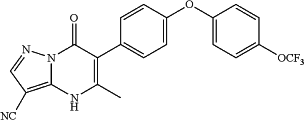
5-methyl-7-oxo-6-(4-(4-(trifluoromethoxy)phenoxy)phenyl)-4,7-dihydropyrazolo[1,5-a]pyrimidine-3-carbonitrile;
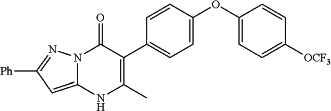
5-methyl-2-phenyl-6-(4-(4-(trifluoromethoxy)phenoxy)phenyl)pyrazolo[1,5-a]pyrimidin-7(4H)-one;
or a stereoisomer thereof, or a pharmaceutically acceptable salt thereof.
|
|||||||||||||||||||||||||||