| CPC A61K 31/439 (2013.01) [A61K 9/0053 (2013.01); A61K 9/08 (2013.01); A61K 9/1611 (2013.01); A61K 9/1623 (2013.01); A61K 9/1641 (2013.01); A61K 9/1652 (2013.01); A61K 9/1682 (2013.01); A61K 9/2009 (2013.01); A61K 9/2018 (2013.01); A61K 9/2031 (2013.01); A61K 9/2054 (2013.01); A61K 9/4825 (2013.01); A61K 31/498 (2013.01); A61K 47/02 (2013.01); A61K 47/10 (2013.01); A61K 47/12 (2013.01); A61K 47/26 (2013.01); A61K 47/38 (2013.01); A61P 35/00 (2018.01)] | 23 Claims |
|
1. A solid formulation comprising a compound of Formula (I):
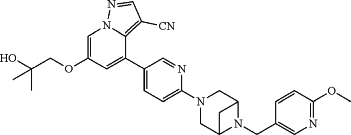 or a pharmaceutically acceptable salt, amorphous form or polymorph form thereof, in an amount from about 20 wt % to about 50 wt % and one or more excipients.
|