| CPC C07D 405/12 (2013.01) [A61K 31/4035 (2013.01); A61K 31/422 (2013.01); A61K 31/4439 (2013.01); A61K 45/06 (2013.01); A61P 11/00 (2018.01); A61P 17/06 (2018.01); A61P 35/00 (2018.01); A61P 37/00 (2018.01); C07B 59/002 (2013.01); C07D 405/14 (2013.01); C07D 413/12 (2013.01); C07B 2200/05 (2013.01)] | 9 Claims |
|
1. A method of assaying a compound for CXCR2 antagonistic activity, said method comprising
(a) contacting the compound with cells expressing CXCR2 and a radioactive CXCR2 ligand to form a reaction mixture;
(b) transferring the reaction mixture onto a GF/B glass filter pre-soaked in a polyethyleneimine solution;
(c) measuring the amount radioactivity remaining on the GF/B glass filter, wherein said method comprises performing steps (a)-(c) with a positive control sample having a formula represented by the structure
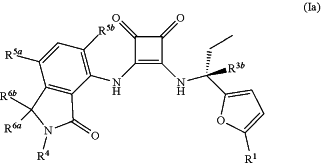 or a pharmaceutically acceptable salt thereof, wherein
R1 is selected from the group consisting of Cl and CH3;
R3b is selected from the group consisting of H and D;
R4 is a member selected from the group consisting of H and C1-8 alkyl, wherein the C1-8 alkyl is optionally substituted with —CONRaRb, —OC(O)NRaRb, —NRaC(O)Rb, —NRaC(O)2Rc, —NRaRb, and —ORa, wherein each Ra and Rb is independently selected from hydrogen, C1-4 alkyl, C1-4 hydroxyalkyl and C1-4 haloalkyl, and RC is selected from C1-4 alkyl, C1-4 hydroxyalkyl and C1-4 haloalkyl;
R5a and R5b are each members independently selected from the group consisting of H, F, Cl and CH3;
R6a and R6b are each members independently selected from the group consisting of H, C1-4 alkyl, C1-4 hydroxyalkyl and C1-4 haloalkyl; or optionally R6a and R6b are taken together to form oxo (═O).
|