| CPC A61N 1/375 (2013.01) [A61N 1/086 (2017.08); A61N 1/18 (2013.01); A61N 1/3752 (2013.01); A61N 1/3758 (2013.01); H01R 43/005 (2013.01); H01R 43/18 (2013.01); H01R 43/205 (2013.01); H05K 5/06 (2013.01); A61B 90/50 (2016.02); A61N 1/3754 (2013.01)] | 24 Claims |
|
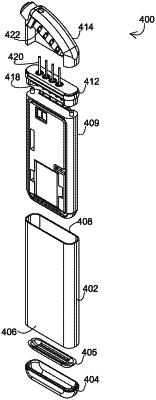
1. An implantable medical device, comprising:
an enclosure sleeve comprising grade 5 titanium, the enclosure sleeve including an enclosure wall with at least a portion of the enclosure wall comprising the grade 5 titanium and having a thickness between 0.007 inches and 0.009 inches, the enclosure sleeve defining a central longitudinal axis, the enclosure sleeve including an open top end and an open bottom end that is opposite the open top end, the enclosure sleeve configured to receive a battery and circuitry that is configured to provide a pulse generator;
a top cap including a feedthrough block, the top cap including a first top cap end portion and a second top cap end portion, the first top cap end portion configured to couple to the open top end of the enclosure sleeve and the second top cap end portion configured to be positioned within the enclosure sleeve with the first top cap end portion extending further from the central longitudinal axis in a direction perpendicular to the central longitudinal axis than the second top cap end portion at each of a first side of the top cap and a second, opposite side of the top cap when the top cap is coupled to the enclosure sleeve; and
a bottom cap configured to couple to the open bottom end of the enclosure sleeve wherein the bottom cap includes a desiccant, the desiccant configured to be a bumper between the bottom cap and a chassis of the implantable medical device.
|