| CPC A61K 31/4745 (2013.01) [A61K 9/0019 (2013.01); A61K 31/381 (2013.01); A61K 31/4045 (2013.01); A61K 31/4462 (2013.01); A61K 31/4535 (2013.01); A61K 31/7048 (2013.01); A61K 47/542 (2017.08); A61K 47/60 (2017.08); A61K 47/61 (2017.08)] | 13 Claims |
|
1. A method for treating a disease or condition related to dopamine insufficiency in the peripheral or central nervous system in a subject, the method comprising the step of administering to the subject a pharmaceutical composition comprising a therapeutically effective amount of a poly(oxazoline) polymer conjugate comprising a water soluble poly(oxazoline) polymer and an agent, wherein a release profile of the agent is selectable based on the selection of the poly(oxazoline) polymer conjugate, the poly(oxazoline) polymer conjugate having the structure:
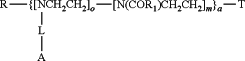 wherein
L is
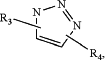 R3 forms a linkage with the poly(oxazoline) polymer;
R4 is —CH2—C(O)—O—, —CH(CH3)—C(O)—O—, —CH2—CH2—C(O)—O—, —CH2—CH2—CH2—C(O)—O—, —CH2—O—C(O)—, —CH2(CH3)—O—C(O)—, —CH2—CH2—O—C(O)— or —CH2—CH2—CH2—O—C(O)—;
R is an initiating group;
R1 is a non-reactive group;
A is the agent, and wherein A is selected from the group consisting of a dopamine agonist, dopamine antagonist, an adenosine A2A antagonist, an anticholinergic, a monamine oxidase-B inhibitor or a catechol-O-methyl transferase inhibitor;
a is ran which indicates a random copolymer or block which indicates a block copolymer;
o is from 1-50;
m is from 1-1000; and
T is a terminating group, wherein the release profile is dependent on the selection of R4.
|