| CPC H10K 85/654 (2023.02) [H10K 50/11 (2023.02); H10K 50/15 (2023.02); H10K 50/16 (2023.02); H10K 50/171 (2023.02); H10K 50/18 (2023.02); H10K 85/615 (2023.02); H10K 85/631 (2023.02); H10K 85/633 (2023.02); H10K 85/636 (2023.02); H10K 85/6572 (2023.02); H10K 50/125 (2023.02); H10K 50/17 (2023.02); H10K 50/81 (2023.02); H10K 50/82 (2023.02); H10K 85/626 (2023.02); H10K 2101/10 (2023.02)] | 1 Claim |
|
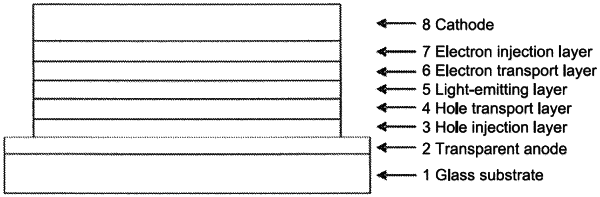
1. An organic electroluminescence element including at least an anode, a hole injection layer, a hole transport layer, a light-emitting layer, an electron transport layer, and a cathode in the stated order,
wherein the hole injection layer contains an electron acceptor and an arylamine compound represented by the following general formula (1),
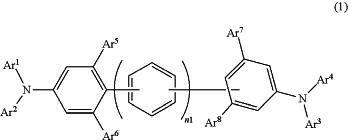 where, in general formula (1),
Ar1 to Ar5 may be the same or differ, and each represents an aromatic hydrocarbon group, an aromatic heterocyclic group, or a fused polycyclic aromatic group, and each of Ar1 to Ar5 excludes a carbazolyl group having a naphthyl group,
Ar6 represents a hydrogen atom, an aromatic hydrocarbon group, an aromatic heterocyclic group, or a fused polycyclic aromatic group,
Ar7 and Ar8 each represents a hydrogen atom,
Ar3 and Ar4 may be bonded to each other via a single bond, a substituted or unsubstituted methylene group, an oxygen atom, or a sulfur atom to form a ring, and
Ar3 or Ar4 may be bonded, via a single bond, a substituted or unsubstituted methylene group, an oxygen atom, or a sulfur atom, to a benzene ring to which a —NAr3Ar4 group is bonded, thereby forming a ring,
wherein the electron acceptor of the hole injection layer comprises at least one selected from the group consisting of tris(bromophenylamine) hexachloroantimony, tetracyanoquinone dimethane, 2,3,5,6-tetrafluoro-tetracyano-1,4-benzoquinone dimethane, and radialene derivatives,
wherein n1 represents 1 or 2, and
wherein each of Ar1 to Ar4 is other than a phenyl group substituted with a methyl.
|