| CPC G06F 8/656 (2018.02) [A61M 5/142 (2013.01); A61M 5/14244 (2013.01); A61M 5/14248 (2013.01); A61M 5/16831 (2013.01); A61M 5/172 (2013.01); G06F 3/04847 (2013.01); G06F 3/04883 (2013.01); G06F 8/61 (2013.01); G06F 8/65 (2013.01); G06F 21/305 (2013.01); G06F 21/31 (2013.01); G06F 21/6245 (2013.01); G06F 21/84 (2013.01); G08B 21/0453 (2013.01); G08B 21/18 (2013.01); G16H 10/60 (2018.01); G16H 20/17 (2018.01); G16H 40/40 (2018.01); G16H 40/60 (2018.01); G16H 40/67 (2018.01); G16H 50/30 (2018.01); G16H 80/00 (2018.01); H04L 9/088 (2013.01); H04L 9/30 (2013.01); H04L 63/101 (2013.01); H04L 67/34 (2013.01); H04W 76/10 (2018.02); H04W 76/14 (2018.02); A61B 5/14532 (2013.01); A61M 2005/14208 (2013.01); A61M 5/1723 (2013.01); A61M 2005/1726 (2013.01); A61M 2205/18 (2013.01); A61M 2205/3327 (2013.01); A61M 2205/3546 (2013.01); A61M 2205/3553 (2013.01); A61M 2205/3584 (2013.01); A61M 2205/3592 (2013.01); A61M 2205/50 (2013.01); A61M 2205/502 (2013.01); A61M 2205/505 (2013.01); A61M 2205/52 (2013.01); A61M 2205/581 (2013.01); A61M 2205/582 (2013.01); A61M 2205/583 (2013.01); A61M 2205/609 (2013.01); A61M 2230/201 (2013.01); G08B 25/00 (2013.01); G16H 40/00 (2018.01)] | 14 Claims |
|
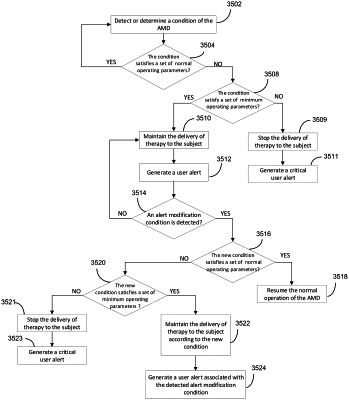
1. A computer-implemented method of managing a non-critical malfunction of a glucose level control system configured to generate a dose output for administration of medicament to a subject, the computer-implemented method comprising:
by a processor of a glucose level control system configured to generate a dose output for administration of medicament to a subject,
detecting a device condition of the glucose level control system; determining that the device condition does not satisfy a set of normal
operating parameters for the glucose level control system indicating an occurrence of a malfunction of the glucose level control system, wherein the set of normal operating parameters enable the glucose level control system to provide a set of therapy related features supported by the glucose level control system;
determining that the device condition satisfies a set of minimum operating parameters, wherein the set of minimum operating parameters are sufficient to permit the glucose level control system to generate the dose output, wherein the set of minimum operating parameters differs from the set of normal operating parameters, and wherein the set of minimum operating parameters enable the glucose level control system to provide a subset of the set of therapy related features supported by the glucose level control system; and
responsive to determining that the device condition does not satisfy the set of normal operating parameters and determining that the device condition satisfies the set of minimum operating parameters:
maintaining the generation of the dose output by the glucose level control system; and
based at least m part on a severity level of the malfunction, generating a non-critical malfunction alert associated with the occurrence of the malfunction, wherein the non-critical malfunction alert enables a user to determine the device condition.
|