| CPC G01N 33/54373 (2013.01) [G01N 33/80 (2013.01)] | 17 Claims |
|
1. A portable blood typing device comprising:
an enclosure having a proximal end, a distal end, and a hollow interior;
an actuatable plunger positioned within the enclosure, the plunger dividing the hollow interior into a first distal chamber and a second proximal chamber;
a closeable aperture on the distal end of the enclosure providing access to the first chamber; and
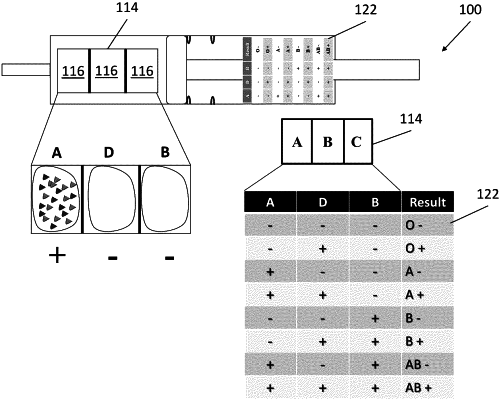
at least one well embedded in the enclosure;
wherein the at least one well comprises at least three distinct surface regions, each surface region comprising a probe that binds to a distinct antigen, a first surface region coated in a first probe that binds to antigen A, a second surface region coated in a second probe that binds to antigen D, and a third surface region coated in a third probe that binds to antigen B.
|