| CPC G01N 31/16 (2013.01) [G01N 1/28 (2013.01); G01N 1/34 (2013.01); G01N 1/4077 (2013.01)] | 5 Claims |
|
1. A detection method for determining chloride ions content in sea sand, characterized by being performed in the steps as follows:
Step 1, preparing a washed filtrate:
drying a sea sand sample to be detected to constant weight, weighing the dried sea sand sample to be detected with a mass of G0, then adding the dried sea sand sample to boiling deionized water, fully stirring for 3-6 min, letting stand for 10-15 min, and then filtering to obtain washed sea sand and a washed filtrate, wherein the deionized water is heated during stirring to keep the temperature not lower than 90° C., and a mass ratio of the dried sea sand sample to be detected for mixing and stirring to the deionized water is 1:(1.5-2.5);
Step 2, preparing a powder filtrate:
drying the washed sea sand from Step 1 until the surface of the sea sand is free of water, then grinding the washed sea sand into powder with a fineness of not less than 100 meshes, then adding the powder to deionized water, fully stirring for 5-10 min, and afterward, filtering to obtain a powder filtrate, wherein a mass ratio of the dried washed sea sand to the deionized water is 1:(1.5-2.5);
Step 3, preparing a mixed filtrate:
firstly, placing the washed filtrate prepared in Step 1 and the powder filtrate prepared in Step 2 in a thermostatic chamber, and letting stand for 15-20 min; then dividing the washed filtrate from Step 1 after standing into 2 equal parts separately, dividing the powder filtrate prepared in Step 2 after standing into 2 equal parts separately, and then mixing one part of the washed filtrate and one part of the powder filtrate to obtain a mixed filtrate, wherein the remaining part of the washed filtrate and the remaining part of the powder filtrate are for later separate use respectively;
Step 4, measuring the mass of chloride ions in different filtrates:
taking the mixed filtrate, the remaining part of the washed filtrate and the remaining part of the powder filtrate from Step 3 in same volume, and measuring the mass H1 of chloride ions in the remaining part of washed filtrate, the mass H2 of chloride ions in the remaining part of powder filtrate and the mass H3 of the chloride ions in the mixed filtrate by using a silver nitrate titration method;
Step 5, determining the chloride ions content in the sea sand:
firstly, calculating the value of a, wherein a is the ratio of a sum of the mass H1 of chloride ions in the remaining part of washed filtrate as measured in Step 4 and the mass H2 of chloride ions in the remaining part of powder filtrate as measured in Step 4 to the mass H3 of chloride ions in the mixed filtrate as measured in Step 4, i.e., a=(H1+H2)/H3;
then, when 0.850≤a≤1.150, directly determining the chloride ions content Q in the sea sand according to the following three conditions; when the value of a is less than 0.850 or the value of a is greater than 1.150, repeating Step 4 and recalculating the value of a until 0.850≤a≤1.150, and then determining the chloride ions content Q in the sea sand according to the following three conditions:
Condition 1: when 0.850≤a<0.975, the total mass H of chloride ions in the sea sand is: H=m(H1+H2)+H3; and then the chloride ions content Q in the sea sand is:
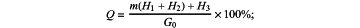 Condition 2: when 0.975≤a≤1.025, the total mass H of chloride ions in the sea sand is: H=H1+H2+H3; and then the chloride ions content Q in the sea sand is:
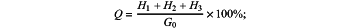 Condition 3: when 1.025<a≤1.150, the total mass H of chloride ions in the sea sand is: H=H1+H2+nH3; and then the chloride ions content Q in the sea sand is:
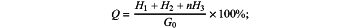 wherein m and n are coefficients, with m=1+(1−a)=2−a, and n=1+(a−1)=a.
|