| CPC C25D 9/12 (2013.01) [B32B 9/00 (2013.01); B32B 15/04 (2013.01); B32B 15/043 (2013.01); B32B 15/08 (2013.01); B32B 15/18 (2013.01); C25D 5/48 (2013.01); C25D 7/00 (2013.01); C25D 7/0614 (2013.01); C25D 9/04 (2013.01); C25D 9/06 (2013.01); C25D 9/08 (2013.01); C25D 9/10 (2013.01); C25D 11/36 (2013.01); C25D 3/30 (2013.01); C25D 5/36 (2013.01)] | 3 Claims |
|
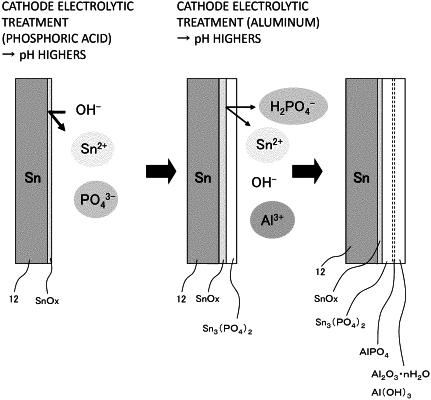
1. A method for producing a surface-treated steel sheet comprising:
forming a phosphate compound layer on a tin-plated steel sheet by means of cathode electrolytic treatment, wherein the tin-plated steel sheet is obtained by tin-plating a steel sheet; and
forming an aluminum-oxygen compound layer on the phosphate compound layer by means of electrolytic treatment using an electrolytic treatment liquid containing aluminum,
wherein the electrolytic treatment liquid has a phosphate content of 0.11 g/L or less in terms of phosphorus atom and an aluminum ion content of 1 to 10 g/L in terms of aluminum atom,
the electrolytic treatment for forming the aluminum-oxygen compound layer is performed by an intermittent electrolysis method where a cycle of energization and stop of energization is repeated, and
a total energization time for forming the aluminum-oxygen compound layer is 1.5 seconds or less.
|