| CPC C12Q 1/6869 (2013.01) [C12Q 1/6823 (2013.01)] | 5 Claims |
|
1. A plurality of different deoxyribonucleic acids covalently immobilized on a solid support, wherein at least one of the plurality different deoxyribonucleic acids comprises a polymerase-incorporated labeled nucleotide analogue derived from the group consisting of labeled nucleotide analogues A-D, and wherein at least one of the plurality of different deoxyribonucleic acids comprises a polymerase-incorporated un-labeled nucleotide analogue derived from the group consisting of un-labeled nucleotide analogues A′-D′
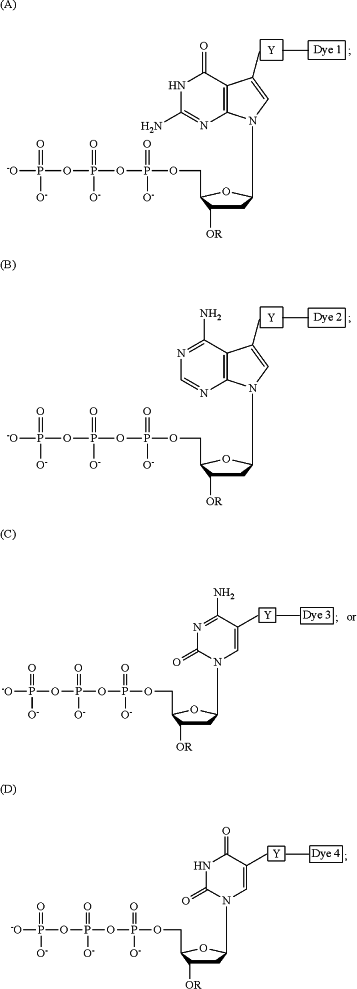 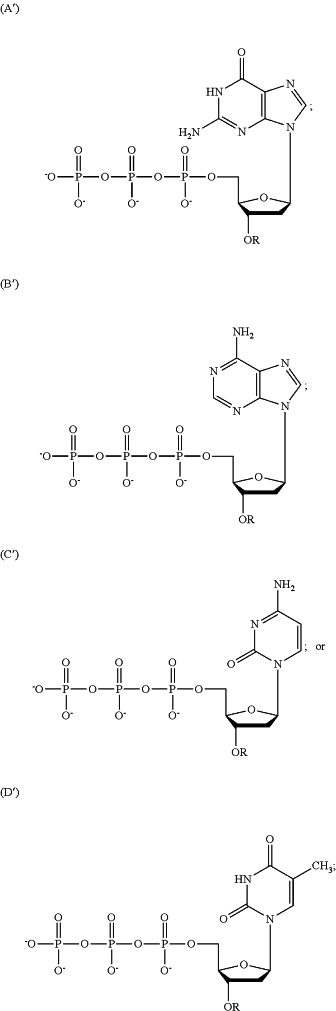 wherein R (a) represents a cleavable, chemical group capping the oxygen at the 3′ position of the deoxyribose of the deoxyribonucleotide analogue, (b) does not interfere with recognition of the analogue as a substrate by the DNA polymerase or with incorporation of the analogue into the growing DNA strand during the DNA polymerase reaction, and (c) is stable during the DNA polymerase reaction;
wherein the covalent bona between the 3′-oxygen and R is stable during the DNA polymerase reaction;
wherein each of Dye 1, Dye 2, Dye 3 and Dye 4 represents a distinguishable, detectable moiety;
wherein (i) the nucleotide analogue is incorporated into the growing DNA strand as a result of the DNA polymerase reaction, (ii) the incorporated analogue is identified, and (iii) the covalent bond between the 3′-oxygen and R is cleaved under conditions compatible with DNA sequencing to allow the incorporation and detection of the next nucleotide analogue;
wherein Y represents a cleavable, chemical linker which (a) does not interfere with recognition of the analogue as a substrate by the DNA polymerase or with incorporation of the analogue into the growing DNA strand during the DNA polymerase reaction and (b) is stable during the DNA polymerase reaction;
wherein the nucleotide analogue:
i) is recognized as a substrate by the DNA polymerase for incorporation into the growing′ DNA strand during the DNA polymerase reaction,
ii) is efficiently and accurately incorporated at the end of the growing DNA strand during the DNA polymerase reaction,
iii) produces a 3′—OH group on the deoxyribose upon cleavage of R under conditions compatible with DNA sequencing, and
iv) no longer includes a detectable moiety on the base upon cleavage of Y under conditions compatible with DNA sequencing;
and wherein if the nucleotide analogue is: (A) or (A′), it forms hydrogen bonds with cytosine or a cytosine nucleotide analogue; (B) or (B′), it forms hydrogen bonds with thymine or a thymine nucleotide analogue; (C) or (C′), it forms hydrogen bonds with guanine or a guanine nucleotide analogue, or (D) or (D′), it forms hydrogen bonds with adenine or an adenine nucleotide analogue.
|