| CPC C12N 9/0071 (2013.01) [A61K 9/0019 (2013.01); A61K 9/5123 (2013.01); A61K 48/0075 (2013.01); A61P 3/00 (2018.01); C12Y 114/16001 (2013.01)] | 19 Claims |
|
1. A method of treating phenylketonuria in a human subject that has phenylketonuria, comprising administering to the human subject an effective amount of a pharmaceutical composition comprising a lipid nanoparticle comprising
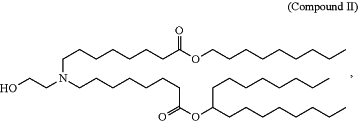 or a salt thereof, wherein the pharmaceutical composition comprises an mRNA comprising an open reading frame encoding a phenylalanine hydroxylase polypeptide.
|