| CPC C12N 5/0634 (2013.01) [A61K 48/0058 (2013.01); C07K 14/00 (2013.01); C07K 16/00 (2013.01); C12N 5/0635 (2013.01); C12N 9/22 (2013.01); C12N 15/11 (2013.01); C12N 15/90 (2013.01); A61K 35/17 (2013.01); C07K 2317/14 (2013.01); C07K 2317/21 (2013.01); C12N 15/113 (2013.01); C12N 2310/20 (2017.05); C12N 2501/056 (2013.01); C12N 2501/2302 (2013.01); C12N 2501/2306 (2013.01); C12N 2501/231 (2013.01); C12N 2501/2315 (2013.01); C12N 2501/24 (2013.01); C12N 2501/52 (2013.01); C12N 2506/11 (2013.01); C12N 2510/00 (2013.01); C12N 2510/02 (2013.01); C12N 2750/14143 (2013.01); C12N 2800/80 (2013.01)] | 15 Claims |
|
1. A method of making a population of plasma cells or plasma cell precursors edited to express a polypeptide, the method comprising:
(a) isolating primary B cells;
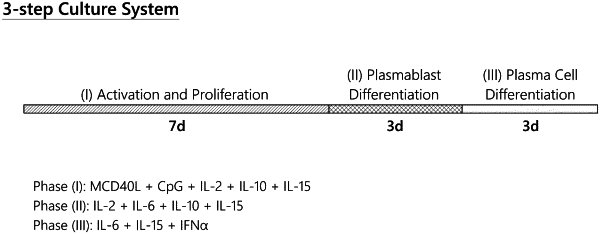
(b) activating the primary B cells in a culture medium for a period of time, so that a population of activated B cells is obtained, wherein the period of time is 7 days and the culture medium comprises (i) oligomerized CD40 ligand comprising two linked CD40L trimers, and (ii) CpG oligodeoxynucleotide, IL-2, IL10 and IL15;
(c) during the period of time, editing the cells by contacting them with:
(i) a ribonucleoprotein (RNP) complex comprising a Cas protein complexed with a guide RNA directed to a target site in a target locus, and
(ii) a nucleic acid including a sequence that encodes the polypeptide and is flanked by homologous sequences to a target site in the target locus, so that the encoding sequence integrates at the target locus, and an edited cell population is obtained that comprises cells which express the polypeptide; and
(d) expanding and differentiating the edited B cell population, thereby producing an edited plasma cell population or plasma cell precursor population characterized in that cells of the population express the polypeptide, wherein the differentiating comprises (i) contacting the edited B cell population with IL-2, IL-6, IL-10 and IL-15 for an initial 3 days to produce the edited plasma cell precursor population, and (ii) contacting the edited plasma cell precursor population with IL-6, IL-15 and IFNα for a further 3 days to produce the edited plasma cell population.
|