| CPC C08J 3/075 (2013.01) [A61K 8/042 (2013.01); A61K 8/73 (2013.01); A61K 8/735 (2013.01); A61K 9/0019 (2013.01); A61Q 19/00 (2013.01); C07C 209/62 (2013.01); C07C 213/00 (2013.01); C07C 269/06 (2013.01); C07F 7/083 (2013.01); C08B 37/0063 (2013.01); C08B 37/0069 (2013.01); C08B 37/0072 (2013.01); C08J 3/24 (2013.01); C08J 7/14 (2013.01); C08K 5/09 (2013.01); C08L 5/00 (2013.01); C08L 5/08 (2013.01); C07C 2603/18 (2017.05); C08J 2305/00 (2013.01); C08J 2305/08 (2013.01)] | 20 Claims |
|
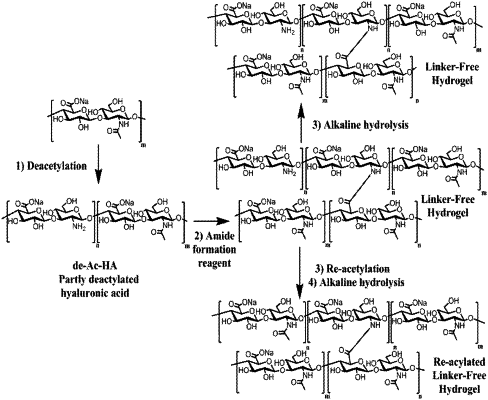
1. A hydrogel product comprising acylated crosslinked glycosaminoglycans (GAGs), produced by a method comprising:
crosslinking (i) an at least partially deacetylated GAG comprising free amine groups and (ii) a second GAG comprising activated carboxyl groups, by forming amide bonds between 2′-amino groups on the at least partially deacetylated GAG and the activated carboxyl groups of the second GAG,
wherein the hydrogel product has residual free amine groups and a degree of acetylation of at least 95%.
|