| CPC C07H 19/056 (2013.01) [A61K 31/7052 (2013.01); C07H 19/23 (2013.01); C07H 19/24 (2013.01)] | 16 Claims |
|
1. A method for treatment of a disorder relating to the binding of a galectin-3 to a ligand in a mammal, wherein a therapeutically effective amount of at least one compound of formula (1) is administered to a mammal in need of said treatment, wherein said compound of formula (1) is a D-galactopyranose compound of formula (1)
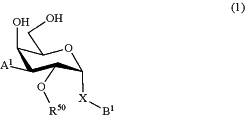 wherein
the pyranose ring is α-D-galactopyranose,
A1 is selected from
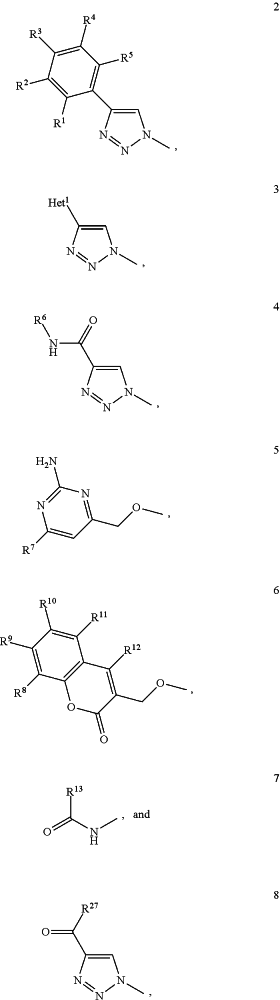 wherein Het1 is selected from a five or six membered heteroaromatic ring, optionally substituted with a group selected from Br; F; Cl; I; CN; NR19R20, wherein R19 and R20 are independently selected from H, C1-3 alkyl, cyclopropyl, iso-propyl, —C(═O)—R21, wherein R21 is selected from H and C1-3 alkyl; C1-3 alkyl, optionally substituted with a F; cyclopropyl, optionally substituted with a F; iso-propyl, optionally substituted with a F; O-cyclopropyl optionally substituted with a F; O-isopropyl optionally substituted with a F; OC1-3 alkyl optionally substituted with a F; and SC1-3 alkyl optionally substituted with a F;
wherein R1-R5 are independently selected from H, CN, NH2, Br, Cl, I, F, methyl optionally substituted with a F, SCH3 optionally substituted with a F, and OCH3 optionally substituted with a F;
wherein R6 is selected from C1-6 alkyl optionally substituted with a halogen, branched C3-6 alkyl and C3-7 cycloalkyl;
wherein R7 is selected from a five or six membered heteroaromatic ring, optionally substituted with a group selected from Br, F, Cl, methyl optionally substituted with a F, and OCH3 optionally substituted with a F, and a phenyl optionally substituted with a group selected from Br, F, Cl, methyl optionally substituted with a F, and OCH3 optionally substituted with a F;
wherein R8-R12 are independently selected from H, F, methyl optionally substituted with a F, and OCH3 optionally substituted with a F;
wherein R13 is a five or six membered heteroaromatic ring optionally substituted with a group selected from H, OH, F, methyl optionally substituted with a F, and OCH3 optionally substituted with a F, a phenyl or a naphthyl, optionally substituted with a group selected from H, OH, F, methyl optionally substituted with a F, and OCH3 optionally substituted with a F;
X is selected from S, SO, SO2, O, C═O, and CR7R8 wherein R7 and R8 are independently selected from hydrogen, OH, or halogen;
wherein R27 is selected from a C1-6 alkyl, branched C3-6 alkyl, C1-6 alkoxy and branched C3-6 alkoxy;
B1 is selected from a pyridinyl, optionally substituted with a group selected from a halogen; a N (2 oxa)-6-azaspiro[3.3]heptanyl; CN; —COOH; —CONR24R25, wherein R24 and R25 are independently selected from H, C1-3 alkyl, cyclopropyl, and iso-propyl or R24 and R25 together with the nitrogen may form a heterocycloalkyl; C1-3 alkyl, optionally substituted with a F; cyclopropyl, optionally substituted with a F; isopropyl, optionally substituted with a F; OC1-3 alkyl, optionally substituted with a F; O-cyclopropyl, optionally substituted with a F; O-isopropyl, optionally substituted with a F; NR30R31, wherein R30 and R31 are independently selected from H, C1-3 alkyl and isopropyl; OH; R18—CONH— wherein R18 is selected from C1-3 alkyl and cyclopropyl; a pyrimidinyl optionally substituted with a substituent selected from CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R71—CONH— wherein R71 is selected from C1-3 alkyl and cyclopropyl; a pyridinyl optionally substituted with a substituent selected from CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R72—CONH— wherein R72 is selected from C1-3 alkyl and cyclopropyl; a tetrahydropyridinyl optionally substituted with a substituent selected from CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R73—CONH— wherein R73 is selected from C1-3 alkyl and cyclopropyl; a pyrrolinyl optionally substituted with a substituent selected from CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R74—CONH— wherein R74 is selected from C1-3 alkyl and cyclopropyl; an oxazolyl optionally substituted with a substituent selected from CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R75—CONH— wherein R75 is selected from C1-3 alkyl and cyclopropyl; a thiazolyl optionally substituted with a substituent selected from CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R76—CONH— wherein R76 is selected from C1-3 alkyl and cyclopropyl; and a C2-4 alkynyl;
R50 is selected from the group consisting of a) C1-6 alkyl optionally substituted with one or more halogen, CN, OR51, NR52R53, and CONH2, wherein R51 is selected from the group consisting of H, CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R54—CONH— wherein R54 is selected from C1-3 alkyl and cyclopropyl, R52 is selected from the group consisting of H, CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R55—CONH— wherein R55 is selected from C1-3 alkyl and cyclopropyl, and R53 is selected from the group consisting of H, CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R56—CONH— wherein R56 is selected from C1-3 alkyl and cyclopropyl, b) branched C3-6 alkyl optionally substituted with one or more halogen, CN, OR57, NR58R59, and CONH2, wherein R57 is selected from the group consisting of H, CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R60—CONH— wherein R60 is selected from C1-3 alkyl and cyclopropyl, R58 is selected from the group consisting of H, CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R61—CONH— wherein R61 is selected from C1-3 alkyl and cyclopropyl, and R59 is selected from the group consisting of H, CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R62—CONH— wherein R62 is selected from C1-3 alkyl and cyclopropyl, and c) cyclic C3-6 alkyl optionally substituted with one or more halogen, CN, OR63, NR64R65, and CONH2, wherein R63 is selected from the group consisting of H, CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R66—CONH— wherein R66 is selected from C1-3 alkyl and cyclopropyl, R64 is selected from the group consisting of H, CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R67—CONH— wherein R67 is selected from C1-3 alkyl and cyclopropyl, and R65 is selected from the group consisting of H, CN, a halogen, methyl optionally substituted with a F, OCH3 optionally substituted with a F, OCH2CH3 optionally substituted with a F, OH, and R68—CONH— wherein R68 is selected from C1-3 alkyl and cyclopropyl; or
a pharmaceutically acceptable salt thereof,
wherein the disorder is selected from the group consisting of: inflammation; fibrosis selected from pulmonary fibrosis, liver fibrosis, kidney fibrosis, ophthalmological fibrosis, fibrosis of the skin, and fibrosis of the heart; scarring; keloid formation; aberrant scar formation; surgical adhesions; septic shock; cancers selected from carcinomas, sarcomas, leukemias, lymphomas, and metastasising cancers; autoimmune diseases selected from psoriasis, rheumatoid arthritis, Crohn's disease, ulcerative colitis, ankylosing spondylitis, and systemic lupus erythematosus; metabolic disorders; heart disease; heart failure; pathological angiogenesis, ocular angiogenesis or a disease or condition associated with ocular angiogenesis; neovascularization related to cancer; eye diseases selected from age-related macular degeneration and corneal neovascularization; atherosclerosis; metabolic diseases; diabetes; type 2 diabetes; insulin resistens; obesity; Diastolic HF; interstitial lung diseases selected from asthma, Hermansky-Pudlak syndrome, and mesothelioma; and liver disorders selected from non-alcoholic steatohepatitis and non-alcoholic fatty liver disease.
|