| CPC C07F 9/6561 (2013.01) [A61K 45/06 (2013.01)] | 45 Claims |
|
1. A compound of Formula I:
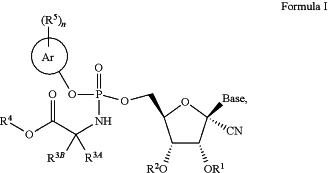 or a pharmaceutically acceptable salt thereof, wherein
each R1 and R2 is independently H, —(CO)C1-C6 alkyl or —(CO)OC1-C6 alkyl, wherein the —(C(O)C1-C6 alkyl or —(CO)OC1-C6 alkyl is optionally substituted with a NH2 group; or
R1 and R2 are combined to form —CO—, —CO—CO—, or —C(O)—C(R1A)(R1B)—C(O)—;
wherein each R1A and R1B is independently H or C1-C6 alkyl;
R3A is H or C1-C6 alkyl; wherein the C1-C6 alkyl is optionally substituted with a —OH or phenyl;
R3B is H or C1-C3 alkyl; and
R4 is (i) C1-C8 alkyl, (ii) (CR8R9CR10R11O)mR12, (iii) C3-C10 cycloalkyl, (iv) 4 to 6 membered heterocyclyl having 1 to 3 heteroatoms independently selected from N, O and S, or (v) 5 to 6 membered heteroaryl having 1 to 3 heteroatoms independently selected from N, O and S; wherein the C1-C8 alkyl, C3-C10 cycloalkyl, 4 to 6 membered heterocyclyl, or 5 to 6 membered heteroaryl is optionally substituted with one or two R4A groups; wherein
each R4A is independently C1-C3 alkyl, C1-C3 alkoxy, C1-C3 haloalkyl, C3-C10 cycloalkyl, C6-C10 aryl, or 4 to 6 membered heterocyclyl having 1 to 3 heteroatoms independently selected from N, O and S; wherein the C3-C10 cycloalkyl, C6-C10 aryl, or 4 to 6 membered heterocyclyl is optionally substituted with one or two substituents independently selected from the group consisting of C1-C6 alkyl, halo, C1-C6 haloalkyl, and C1-C6 alkoxy;
Base is
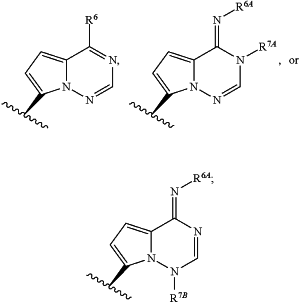 wherein
R6 is —N(H)R6A; and
each R6A, R7A and R7B is independently H or —CH2OP(O)(OH)2;
Ar is C6-C10 aryl or 5 to 10 membered heteroaryl containing one, two, or three heteroatoms selected from the group consisting of O, N, and S;
n is 0, 1, 2, or 3;
each R5 is independently halo, cyano, C1-C6 alkyl, C1-C6 haloalkyl, C3-C6 cycloalkyl, C1-C6 alkoxy, C3-C6 cycloalkoxy, —COOR5A, —SO2R5A, 4 to 6 membered heterocycloalkyl containing one, two or three heteroatoms selected from N, O, and S, or 5 to 6 membered heteroaryl containing one, two or three heteroatoms selected from N, O, and S; wherein the C1-C6 alkyl, C1-C6 haloalkyl, C3-C6 cycloalkyl, C3-C6 cycloalkoxy, C1-C6 alkoxy, 4 to 6 membered heterocycloalkyl and 5 to 6 membered heteroaryl is optionally substituted with one or two R5B groups; or
two R5 groups on adjacent carbon atoms are joined to form a C5-C6 cycloalkyl;
each R5A is independently C1-C6 alkyl;
each R5B is independently —OH, —OR5C, —COOR5c and —NHCOOR5D; wherein R5C is C1-C6 alkyl and R5D is C1-C3 alkyl optionally substituted with a phenyl group;
each R8, R9, R10, R11 and R12 is independently H or C1-C3 alkyl;
m is 1, 2, 3, 4, or 5;
provided that when R1 and R2 are both H then:
(i) n is 1, 2, or 3; or
(ii) R4 is C1-C8 alkyl substituted with one or two groups independently selected from C1-C3 alkoxy, C1-C3 haloalkyl, C3-C10 cycloalkyl, C6-C10 aryl, or 4 to 6 membered heterocyclyl having 1 to 3 heteroatoms independently selected from N, O and S;
wherein the C3-C10 cycloalkyl, C6-C10 aryl, or 4 to 6 membered heterocyclyl is optionally substituted with one or two substituents independently selected from the group consisting of C1-C6 alkyl, halo, C1-C6 haloalkyl, and C1-C6 alkoxy; or
(iii) R4 is (a) —(CR8R9CR10R11O)mR12, (b) monocyclic C3-C10 cycloalkyl substituted with substituted with one or two R4A groups, (c) bicyclic C3-C10 cycloalkyl, (d) 4 to 6 membered heterocyclyl having 1 to 3 heteroatoms independently selected from N, O and S, or (e) 5 to 6 membered heteroaryl having 1 to 3 heteroatoms independently selected from N, O and S;
wherein the bicyclic C3-C10 cycloalkyl, 4 to 6 membered heterocyclyl, or 5 to 6 membered heteroaryl is optionally substituted with one or two R4A groups; or
(iv) Base is
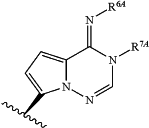 and at least one of R6A and R7A is —CH2OP(O)(OH)2; or Base is
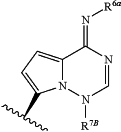 «and at least one of R6A and R7B is —CH2OP(O)(OH)2.
|