| CPC C07D 519/00 (2013.01) [C07D 403/14 (2013.01); C07D 413/14 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 487/04 (2013.01); C07D 513/04 (2013.01)] | 11 Claims |
|
1. A method of treating a STING mediated disease or disorder in a subject in need thereof, comprising administering to the subject a pharmaceutically effective amount of the compound of Formula (V-f1), Formula (V-f2), Formula (V-f3), Formula (V-f4), Formula (V-f5), Formula (V-f6), Formula (V-f7), Formula (V-h1), Formula (V-h2), Formula (V-h3), Formula (V-h4), Formula (V-h5), Formula (V-h6), or Formula (V-h7):
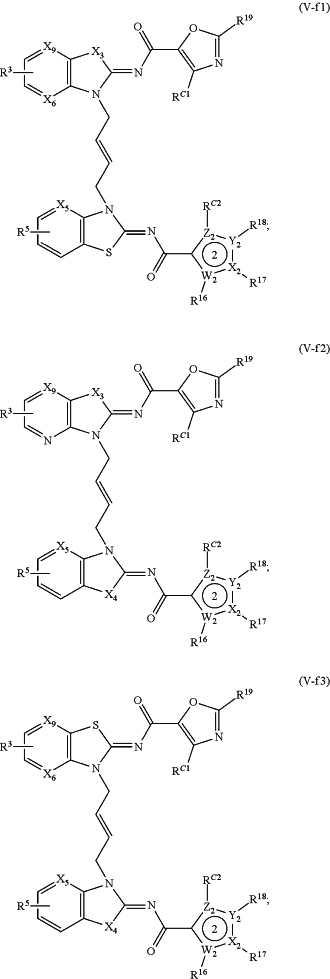 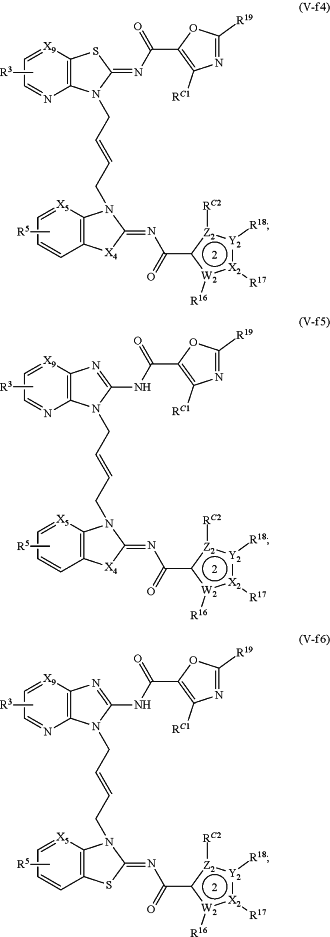 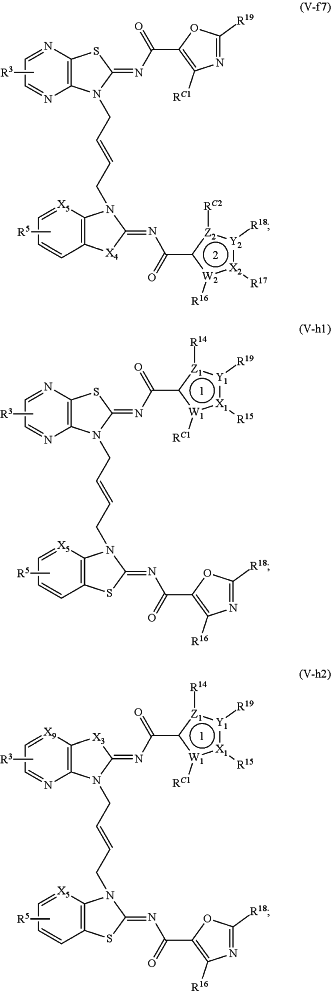 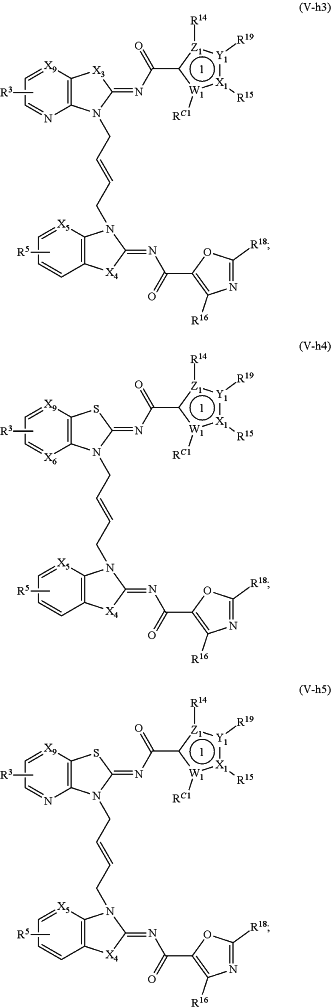 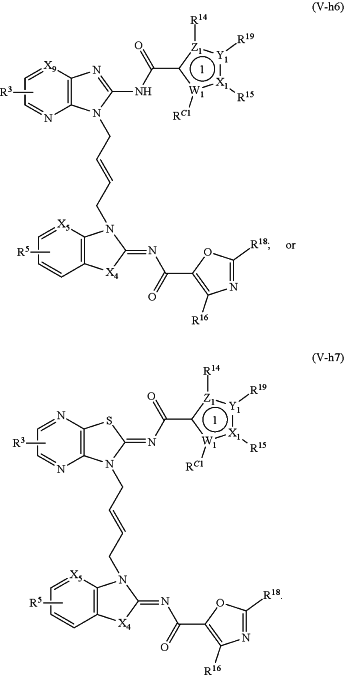 or a prodrug, solvate, pharmaceutically acceptable salt, or tautomer thereof, wherein:
Y1, Z1, Y2, and Z2 are each independently O, S, or N;
X1, W1, X2, and W2 are each independently C or N;
X3 and X4, when present, are each independently S or NW;
X5 is N or CR A2;
X6, when present, is N or CRA1;
X9, when present, is N or CH;
R3 and R5 are each independently —CON(Rd)(Rf), —CH2N(Rd)(Rf), —N(Rd)(Rf), —N(Rd)CO(Rf), —CH2N(Rd)CO(Rf) or one of R3 and R5 is —CON(Rd)(Rf), —CH2N(Rd)(Rf), —N(Rd)(Rf), —N(Rd)CO(Rf) or —CH2N(Rd)CO(Rf), and the other of R3 and R5 is H, —COOH, or —CO2Rc;
Rc is C1-4 alkyl;
RA2 and RA1, when present, are each independently halogen, hydroxyl, optionally substituted (C1-6 alkyl), substituted (C1-6 alkyl)oxy-, optionally substituted (C1-6 alkyl)amino-, or optionally substituted (C1-6 alkyl)(C1-4 alkyl)amino-,
wherein C1-6 alkyl of said optionally substituted (C1-6 alkyl) or substituted (C1-6 alkyl)oxy- is optionally substituted with 1-4 substituents each independently selected from the group comprising hydroxyl, C1-4 alkoxyl, —N(Re)(Rf), —CO2(Rf), —CON(Re)(Rf), and —COOH;
each Rd is independently H, hydroxy, or C1-4 alkyl;
each Re is independently selected from H, (C1-4 alkyl), —CO(C1-4 alkyl), —OCO(C1-4 alkyl), and —CO2(C1-4 alkyl);
each Rf is independently H, hydroxy, or (C1-4 alkyl);
RC2 and R14 are each independently absent or C1-4 alkyl, wherein C1-4 alkyl is optionally substituted by a substituent selected from halogen, —ORc, —NRcRd, —CO2Rc, —CONRcRd, —SO2NRcRd, and —OCONRcRd;
R16 and RC1 are each independently absent, H or C1-4 alkyl; and
R15, R17, R18, or R19 are each independently absent, H, or C1-4 alkyl, wherein C1-4 alkyl is optionally substituted by a substituent selected from halogen, —ORc, —NRcRd, —CO2Rc, —CONRcRd, —SO2NRcRd, and —OCONRcRd.
|