| CPC C07D 487/04 (2013.01) [C07D 491/147 (2013.01); C07D 491/20 (2013.01); C07D 519/00 (2013.01); C07B 2200/05 (2013.01)] | 28 Claims |
|
1. A compound having the structure of Formula (I):
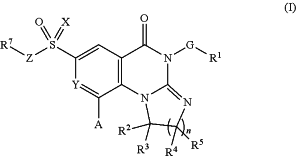 or a pharmaceutically acceptable salt, deuteroisotope, or stereoisomer thereof,
wherein:
Z is —NH—;
R7 is alkyl, cycloalkyl, or 1,1′-bi(cyclopropan)-1-yl;
wherein the alkyl is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl; and
wherein the cycloalkyl or 1,1′-bi(cyclopropan)-1-yl is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl;
X is O;
Y is CH, CF, or N;
A is H, halo, CN, C1-C6 alkyl, alkyl(carbocyclyl), alkyl(heterocyclyl), C2-C6 alkenyl, C2-C6 alkynyl, alkynyl(carbocyclyl), NR9R9, OH, OC1-C6 alkyl, C3-C7 carbocyclyl, heterocyclyl, aryl, or heteroaryl;
wherein the C1-C6 alkyl, alkyl portion of alkyl(carbocyclyl), alkyl portion of alkyl(heterocyclyl), C2-C6 alkenyl, C2-C6 alkynyl, alkynyl portion of alkynyl(carbocyclyl), or OC1-C6 alkyl is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl; and
wherein the carbocyclyl portion of alkyl(carbocyclyl), heterocyclyl portion of alkyl(heterocyclyl), carbocyclyl portion of alkynyl(carbocyclyl), C3-C7 carbocyclyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with one or more substituents independently selected from the group consisting of halo, CN, NO2, alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl;
each R9 is independently H or C1-C6 alkyl, wherein each C1-C6 alkyl is optionally and independently substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl;
G is a bond, cycloalkylene, or heterocycloalkylene, wherein the cycloalkylene or heterocycloalkylene is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl;
R1 is alkyl, alkyl(cycloalkyl), alkyl(heterocyclyl), aralkyl, alkyl(heteroaryl), alkenyl, or alkynyl;
wherein the alkyl, alkyl portion of alkyl(cycloalkyl), alkyl portion of alkyl(heterocyclyl), alkyl portion of aralkyl, alkyl portion of alkyl(heteroaryl), alkenyl, or alkynyl is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl; and
wherein the cycloalkyl portion of alkyl(cycloalkyl), heterocyclyl portion of alkyl(heterocyclyl), aryl portion of aralkyl, or heteroaryl portion of alkyl(heteroaryl) is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl;
R2 is H, halo, CN, alkyl, alkyl(cycloalkyl), alkyl(heterocyclyl), alkenyl, alkynyl, alkynyl(cycloalkyl), OH, cycloalkyl, heterocyclyl, aryl, or heteroaryl;
wherein the alkyl, alkyl portion of alkyl(cycloalkyl), alkyl portion of alkyl(heterocyclyl), alkenyl, alkynyl, or alkynyl portion of alkynyl(cycloalkyl) is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl; and
wherein the cycloalkyl portion of alkyl(cycloalkyl), heterocyclyl portion of alkyl(heterocyclyl), cycloalkyl portion of alkynyl(cycloalkyl), cycloalkyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl;
R3 is H, halo, CN, alkyl, alkyl(cycloalkyl), alkyl(heterocyclyl), alkenyl, alkynyl, alkynyl(cycloalkyl), OH, cycloalkyl, heterocyclyl, aryl, or heteroaryl;
wherein the alkyl, alkyl portion of alkyl(cycloalkyl), alkyl portion of alkyl(heterocyclyl), alkenyl, alkynyl, or alkynyl portion of alkynyl(cycloalkyl) is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl; and
wherein the cycloalkyl portion of alkyl(cycloalkyl), heterocyclyl portion of alkyl(heterocyclyl), cycloalkyl portion of alkynyl(cycloalkyl), cycloalkyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl; or
R2 and R3, taken together with the carbon atom to which they are attached, form a carbocyclyl or heterocyclyl, wherein the carbocyclyl or heterocyclyl is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl;
R4 is H, halo, CN, alkyl, alkyl(cycloalkyl), alkyl(heterocyclyl), alkenyl, alkynyl, alkynyl(cycloalkyl), OH, cycloalkyl, heterocyclyl, aryl, or heteroaryl;
wherein the alkyl, alkyl portion of alkyl(cycloalkyl), alkyl portion of alkyl(heterocyclyl), alkenyl, alkynyl, or alkynyl portion of alkynyl(cycloalkyl) is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl; and
wherein the cycloalkyl portion of alkyl(cycloalkyl), heterocyclyl portion of alkyl(heterocyclyl), cycloalkyl portion of alkynyl(cycloalkyl), cycloalkyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl;
R5 is H, halo, CN, alkyl, alkyl(cycloalkyl), alkyl(heterocyclyl), alkenyl, alkynyl, alkynyl(cycloalkyl), OH, cycloalkyl, heterocyclyl, aryl, or heteroaryl;
wherein the alkyl, alkyl portion of alkyl(cycloalkyl), alkyl portion of alkyl(heterocyclyl), alkenyl, alkynyl, or alkynyl portion of alkynyl(cycloalkyl) is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl; and
wherein the cycloalkyl portion of alkyl(cycloalkyl), heterocyclyl portion of alkyl(heterocyclyl), cycloalkyl portion of alkynyl(cycloalkyl), cycloalkyl, heterocyclyl, aryl, or heteroaryl is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl; or
any R4 and R5, taken together with the carbon atom to which they are attached, independently forms a carbocyclyl or heterocyclyl, wherein each carbocyclyl or heterocyclyl is optionally and independently substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl; or
R3 and R4, taken together with the carbon atoms to which they are attached, form a carbocyclyl or heterocyclyl, wherein the carbocyclyl or heterocyclyl is optionally substituted with one or more substituents independently selected from the group consisting of D, halo, CN, NO2, alkyl, haloalkyl, alkenyl, haloalkenyl, alkynyl, haloalkynyl, C(O)Ra, C(O)N(Ra)2, C(O)ORa, N(Ra)2, NRaC(O)Ra, NRaC(O)ORa, NRaS(O)tRa, ORa, OC(O)Ra, OC(O)N(Ra)2, OC(O)ORa, ORcC(O)N(Ra)2, ═O, S(O)tRa, S(O)tN(Ra)2, S(O)tORa, ═S, cycloalkyl, heterocycloalkyl, aryl, and heteroaryl;
each Ra is independently H, D, alkyl, alkyl(cycloalkyl), alkyl(heterocycloalkyl), aralkyl, alkyl(heteroaryl), cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
each Rc is independently alkylene or alkenylene;
n is 1, 2, or 3; and
each t is independently 1 or 2.
|
|
27. A pharmaceutical composition comprising a pharmaceutically acceptable excipient and a compound of claim 1, or a pharmaceutically acceptable salt, deuteroisotope, or stereoisomer thereof.
|