| CPC C07D 487/04 (2013.01) [A61K 47/55 (2017.08); C07D 519/00 (2013.01)] | 13 Claims |
|
1. A compound represented by the following Formula I:
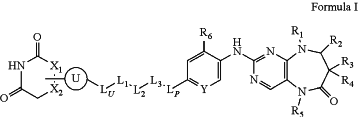 or a pharmaceutically acceptable salt or stereoisomer thereof,
wherein:
X1 is CH, —CH2—, or N;
X2 is CH, —CH2—, or N;
ring U is phenylene or 5- or 6-membered heteroarylene;
wherein the phenylene or 5- or 6-membered heteroarylene is linked to X1 or X2; and
wherein the phenylene or 5- or 6-membered heteroarylene is optionally substituted with one or more independently selected RU substituents;
each RU is independently halo, C1-4 alkyl, C1-4 haloalkyl, C1-4 aminoalkyl, C1-4 hydroxyalkyl, or OC1-4 alkyl;
LU is a bond, —(CH2)x—, —(CH2)xNH—, —(CH2)xO—, —C(O)—, or phenylene;
L1 is a bond or heterocycloalkylene;
wherein the heterocycloalkylene contains at least one nitrogen ring heteroatom; and
wherein the heterocycloalkylene is optionally substituted with one or more substituents independently selected from halo, C1-4 alkyl, C1-4 haloalkyl, OH, OC1-4 alkyl, or ═O;
L2 is —(CH2)y1—, —(CD2)y1-, —(CH2)y2—C(O)—(CH2)y3—, —(CH2)y2—NH—(CH2)y3—, or —(CH2)y2—N(C1-4 alkyl)-(CH2)y3—;
L3 is a bond, cycloalkylene, or heterocycloalkylene;
wherein the heterocycloalkylene contains at least one nitrogen ring heteroatom; and
wherein the cycloalkylene or heterocycloalkylene is optionally substituted with one or more substituents independently selected from halo, C1-4 alkyl, or C1-4 haloalkyl;
LP is —(CH2)p—NH—C(O)— or —(CH2)p—O—C(O)—, wherein the —C(O)— of LP is bonded to the ring bearing R6;
p is 0, 1, or 2;
x is 0, 1, 2, 3, or 4;
y1 is 0, 1, 2, 3, 4, 5, or 6;
y2 is 0, 1, 2, 3, 4, 5, or 6;
y3 is 0, 1, 2, 3, 4, 5, or 6;
z is 0, 1, 2, 3, 4, 5, or 6;
Y is CR7 or N;
R6 is halo, C1-4 alkyl, C1-4 haloalkyl, C1-4 aminoalkyl, C1-4 hydroxyalkyl, or OC1-4 alkyl;
R7 is H or halo;
R1 is C1-4 alkyl;
R2 is H or C1-4 alkyl; or
R1 and R2, taken together with the carbon and nitrogen atoms to which they are attached, form a 5- or 6-membered ring;
R3 is H, halo, or C1-4 alkyl;
R4 is H, halo, or C1-4 alkyl; or
R3 and R4, taken together with the carbon atom to which they are attached, form a 3- to 6-membered ring; and
R5 is C1-4 alkyl.
|