| CPC C07D 471/04 (2013.01) [C07D 407/12 (2013.01); C07D 413/10 (2013.01); C07D 413/14 (2013.01)] | 26 Claims |
|
1. A method of enhancing AMPA receptor function in a mammal, wherein enhancing AMPA receptor function treats at least one psychiatric disease or disorder, comprising administering to a mammal an effective amount of a compound represented by the formula (I):
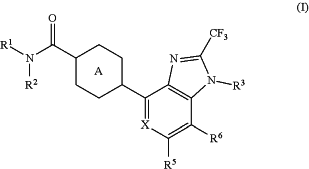 wherein
R1 and R2 are each independently a hydrogen atom or a substituent, or R1 and R2 are bonded to each other to form, together with the adjacent nitrogen atom, a non-aromatic nitrogen-containing heterocycle that is unsubstituted or that is substituted with from one to three substituents independently selected from Substituent Group A,
R3 is a C1-6 alkyl group that is unsubstituted or that is substituted with from one to five substituents independently selected from Substituent Group A,
X is CR4 or N,
R4, R5 and R6 are each independently a hydrogen atom, a halogen atom, a C1-6 alkyl group that is unsubstituted or that is substituted with from one to five substituents independently selected from Substituent Group A or a C1-6 alkoxy group that is unsubstituted or that is substituted with from one to five substituents independently selected from Substituent Group A, and
Ring A is a 6-membered aromatic ring optionally further substituted by 1 to 4 substituents selected from (i) a halogen atom, (ii) a C1-6 alkyl group and (iii) a C1-6 alkoxy group,
or a salt thereof,
wherein Substituent Group A is the group consisting of:
(1) a halogen atom,
(2) a nitro group,
(3) a cyano group,
(4) an oxo group,
(5) a hydroxy group,
(6) a C1-6 alkoxy group,
(7) a C6-14 aryloxy group,
(8) a C7-16 aralkyloxy group,
(9) a 5- to 14-membered aromatic heterocyclyloxy group,
(10) a 3- to 14-membered non-aromatic heterocyclyloxy group,
(11) a C1-6 alkyl-carbonyloxy group,
(12) a C6-14 aryl-carbonyloxy group,
(13) a C1-6 alkoxy-carbonyloxy group,
(14) a mono- or di-C1-6 alkyl-carbamoyloxy group,
(15) a C6-14 aryl-carbamoyloxy group,
(16) a 5- to 14-membered aromatic heterocyclylcarbonyloxy group,
(17) a 3- to 14-membered non-aromatic heterocyclylcarbonyloxy group,
(18) an C1-6 alkylsulfonyloxy group,
(19) a C6-14 arylsulfonyloxy group,
(20) an C1-6 alkylthio group,
(21) a 5- to 14-membered aromatic heterocyclic group,
(22) a 3- to 14-membered non-aromatic heterocyclic group,
(23) a formyl group,
(24) a carboxy group,
(25) an C1-6 alkyl-carbonyl group,
(26) a C6-14 aryl-carbonyl group,
(27) a 5- to 14-membered aromatic heterocyclylcarbonyl group,
(28) a 3- to 14-membered non-aromatic heterocyclylcarbonyl group,
(29) a C1-6 alkoxy-carbonyl group,
(30) a C6-14 aryloxy-carbonyl group,
(31) a C7-16 aralkyloxy-carbonyl group,
(32) a carbamoyl group,
(33) a thiocarbamoyl group,
(34) a mono- or di-C1-6 alkyl-carbamoyl group,
(35) a C6-14 aryl-carbamoyl group,
(36) a 5- to 14-membered aromatic heterocyclylcarbamoyl group,
(37) a 3- to 14-membered non-aromatic heterocyclylcarbamoyl group,
(38) a C1-6 alkylsulfonyl group,
(39) a C6-14 arylsulfonyl group,
(40) a 5- to 14-membered aromatic heterocyclylsulfonyl group,
(41) a C1-6 alkylsulfinyl group,
(42) a C6-14 arylsulfinyl group,
(43) a 5- to 14-membered aromatic heterocyclylsulfinyl group,
(44) an amino group,
(45) a mono- or di-C1-6 alkylamino group,
(46) a mono- or di-C6-14 arylamino group,
(47) a 5- to 14-membered aromatic heterocyclylamino group,
(48) a C7-16 aralkylamino group,
(49) a formylamino group,
(50) a C1-6 alkyl-carbonylamino group,
(51) a (C1-6 alkyl)(C1-6 alkyl-carbonyl) amino group,
(52) a C6-14 aryl-carbonylamino group,
(53) a C1-6 alkoxy-carbonylamino group,
(54) a C7-16 aralkyloxy-carbonylamino group,
(55) a C1-6 alkylsulfonylamino group,
(56) a C6-14 arylsulfonylamino group,
(57) a C1-6 alkyl group optionally substituted by 1 to 3 halogen atoms,
(58) a C2-6 alkenyl group,
(59) a C2-6 alkynyl group,
(60) a C3-10 cycloalkyl group,
(61) a C3-10 cycloalkenyl group, and
(62) a C6-14 aryl group,
to the mammal.
|