| CPC C07D 405/14 (2013.01) [A61K 31/444 (2013.01); A61K 31/506 (2013.01); A61K 31/5377 (2013.01); A61K 31/541 (2013.01); A61K 31/55 (2013.01); A61K 31/553 (2013.01); A61P 35/00 (2018.01); C07D 401/14 (2013.01); C07D 403/04 (2013.01); C07D 403/14 (2013.01); C07D 409/14 (2013.01); C07D 413/14 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 471/08 (2013.01); C07D 487/04 (2013.01); C07D 497/08 (2013.01); C07D 498/22 (2013.01); C07D 513/04 (2013.01)] | 28 Claims |
|
1. A method for:
inhibiting the activity of ERK1/2;
(ii) treating lung cancer, melanoma, or colorectal cancer;
(iii) treating leukemia or lymphoma;
(iv) treating a haematological malignancy or condition of lymphoid lineage or of myeloid lineage;
(v) treating an adenoma or carcinoma; or
(vi) treatment of a disease state or condition selected from tumours of epithelial origin; haematological malignancies and premalignant haematological disorders and disorders of borderline malignancy; tumours of mesenchymal origin; neural crest cell-derived tumours; tumours of the central or peripheral nervous system; endocrine tumours; ocular and adnexal tumours; germ cell and trophoblastic tumours; and paediatric and embryonal tumours; or syndromes which leave the patient susceptible to malignancy;
said method comprising administering to a subject a therapeutically effective amount of a compound selected from:
(a) a compound having the formula (2):
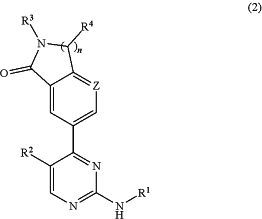 or a pharmaceutically acceptable salt or tautomer thereof; and
(b) a compound having the formula (5):
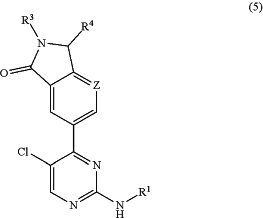 or a pharmaceutically acceptable salt or tautomer thereof;
wherein
n is 1 or 2;
Z is selected from C—Rz and N;
Rz is selected from hydrogen; halogen; methoxy; and C1-3 alkyl optionally substituted with hydroxy or methoxy;
R1 is selected from:
(Alk1)t-Cyc1; wherein t is 0 or 1; and Alk1 is a C1-4 straight chain or branched alkylene group optionally substituted with 1 or 2 hydroxy groups; and
C1-6 acyclic hydrocarbon groups which are unsubstituted or substituted with 1, 2 or 3 substituents R5 selected from hydroxy; oxo; fluorine; and cyano; and wherein 1 or 2 but not all of the carbon atoms of the hydrocarbon group can be replaced by O or N;
Cyc1 is a cyclic group selected from (a) 3 to 9 membered non-aromatic monocyclic and bicyclic carbocyclic and heterocyclic groups containing 0, 1, 2, or 3 heteroatom ring members selected from O, N, S, S(O) and S(O)2; (b) 5 to 6 membered monocyclic heteroaryl groups containing 1, 2 or 3 heteroatom ring members of which 1 is N and the others, when present, are selected from 0, N and S; and (c) 3 to 7 membered monocyclic carbocyclic groups; wherein each cyclic group (a), (b) and (c) is unsubstituted or substituted with 1, 2 or 3 substituents R6 selected from hydroxy; oxo; fluorine; amino; NH(Hyd1); N(Hyd1)2; O-Hyd1; —C(═O)—Hyd1; —C(═O)—O-Hyde and Hyd1; where Hyd1 is a C1-4 non-aromatic hydrocarbon group optionally substituted with one or more substituents selected from fluorine, hydroxyl and methoxy;
R2 is selected from hydrogen; halogen; and C1-3 hydrocarbon groups optionally substituted with one or more fluorine atoms;
R3 is hydrogen or a group L1-R7;
R4 is selected from hydrogen; methoxy; and C1-3 alkyl optionally substituted with hydroxy, amino, mono- or di-C1-2 alkylamino, a cyclic amino group or methoxy; wherein the cyclic amino group is a saturated 4-7 membered heterocyclic group containing a nitrogen ring member and optionally a second heteroatom ring member selected from O, N and S, wherein the cyclic amino group is linked via a nitrogen ring member thereof to the C1-3 alkyl, and wherein the cyclic amino group is optionally substituted with one or two methyl groups; provided that no more than one R4 can be other than hydrogen or methyl;
L1 is selected from a bond; Alk2, Alk2-Oand Alk2-C(═O) wherein Alk2 is a C1-4 straight chain or branched alkylene group which is optionally substituted with one or more substituents selected from hydroxy, methoxy, amino, methylamino, dimethylamino and fluorine;
R7 is selected from:
hydrogen;
CO2H;
NR8R9;
a carbocyclic or heterocyclic group having from 3 to 12 ring members, of which 0, 1, 2 or 3 are heteroatom ring members selected from O, N and S and oxidised forms of S, the carbocyclic or heterocyclic group being optionally substituted with one or more substituents R10; and
an acyclic C1-8 hydrocarbon group optionally substituted with one or more substituents selected from hydroxy; oxo; halogen; cyano; carboxy; amino; mono- or di-C1-4 alkylamino; and carbocyclic and heterocyclic groups having from 3 to 12 ring members, of which 0, 1, 2 or 3 are heteroatom ring members selected from O, N and S and oxidised forms of S, the carbocyclic or heterocyclic group being optionally substituted with one or more substituents R10; wherein one or two but not all of the carbon atoms of the acyclic C1-8 hydrocarbon group may optionally be replaced by O, S, SO, SO2 or NR11;
R8 is selected from hydrogen and a C1-4 hydrocarbon group, the C1-4 hydrocarbon group being optionally substituted with 1-2 substituents selected from hydroxy, amino, mono-C1-4 alkylamino, di-C1-4 alkylamino, and 4-7 membered saturated heterocyclic rings containing 1-2 heteroatom ring members selected from 0 and N, wherein the mono-C1-4 alkylamino, di-C1-4 alkylamino, and 4-7 membered saturated heterocyclic rings are each optionally substituted with 1-2 hydroxy or C1-3 alkyl substituents;
R9 is selected from:
hydrogen;
a carbocyclic or heterocyclic group having from 3 to 12 ring members, of which 0, 1, 2 or 3 are heteroatom ring members selected from O, N and S and oxidised forms of S, the carbocyclic or heterocyclic group being optionally substituted with one or more substituents R10; and
an acyclic C1-8 hydrocarbon group optionally substituted with one or more substituents selected from hydroxy; oxo; halogen; cyano; carboxy; amino; mono- or di-C1-4 alkylamino; and carbocyclic and heterocyclic groups having from 3 to 12 ring members, of which 0, 1, 2 or 3 are heteroatom ring members selected from O, N and S and oxidised forms of S, the carbocyclic or heterocyclic group being optionally substituted with one or more substituents R10; wherein one or two but not all of the carbon atoms of the acyclic C1-8 hydrocarbon group may optionally be replaced by O, S, SO, SO2 or NR11;
or NR8R9 forms a heterocyclic group having from 4 to 12 ring members wherein, in addition to the nitrogen atom of N8R9, the heterocyclic group optionally contains 1 or 2 further heteroatom ring members selected from O, N and S and oxidised forms of S; and wherein the heterocyclic group is optionally substituted with one or more substituents R10;
R10 is selected from:
halogen; hydroxy; oxo; cyano;
OR12 wherein R12 is C1-6 alkyl or C3-6 cycloalkyl, each being optionally substituted with halogen;
an acyclic C1-8 hydrocarbon group optionally substituted with one or more substituents selected from hydroxy; oxo; halogen; cyano; carboxy; amino; mono- or di-C1-4 alkylamino; and carbocyclic and heterocyclic groups having 3 to 7 ring members of which 0, 1, 2, 3 or 4 are heteroatom ring members selected from N, O and S, wherein the carbocyclic and heterocyclic groups are optionally substituted with one or more substituents R13 selected from hydroxy; halogen; cyano; amino; —NH(Hyd1); —N(Hyd1)2; and —(O)v-Hyd1 where v is 0 or 1; wherein one or two but not all of the carbon atoms of the acyclic C1-8 hydrocarbon group may optionally be replaced by O, S, SO, SO2 or NR11; and
carbocyclic and heterocyclic groups having 3 to 7 ring members of which 0, 1, 2, 3 or 4 are heteroatom ring members selected from N, O and S, wherein the carbocyclic and heterocyclic groups are optionally substituted with one or more substituents R13; and
R11 is selected from hydrogen and a C1-4 hydrocarbon group.
|