| CPC C07D 237/28 (2013.01) [C07D 403/08 (2013.01); C07D 405/04 (2013.01)] | 17 Claims |
|
1. A pharmaceutical composition comprising a compound of Formula (I),
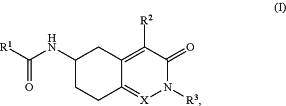 or a pharmaceutically acceptable salt thereof, wherein
R1 is H, optionally substituted C1-C6-alkyl; optionally substituted aryl; optionally substituted C3-C7-cycloalkyl; optionally substituted heterocyclyl; or optionally substituted heteroaryl;
R2 is H, optionally substituted C1-6 alkyl, or OR4 wherein R4 is H or optionally substituted C1-6 alkyl;
R3 is H, optionally substituted C1-C6-alkyl optionally substituted C3-C8-cycloalkyl; optionally substituted aryl; optionally substituted heteroaryl; optionally substituted 3 to 8 membered heterocyclyl; or —(CH2)n—Y, where n is 1-4 and Y is optionally substituted C3-C8-cycloalkyl; optionally substituted heterocyclyl; optionally substituted aryl; optionally substituted heteroaryl; optionally substituted C1-C6-alkoxy; —C(O)OH or —C(O)N(R8)2;
each R8 is independently H or C1-C6-alkyl; and
X is N or CR5; wherein R5 is H or optionally substituted C1-6 alkyl; and
a pharmaceutically acceptable carrier;
wherein
each heteroaryl is selected from the group consisting of pyridinyl, pyrazinyl, pyrimidinyl, pyrrolyl, pyrazolyl, imidazolyl, thiazolyl, oxazolyl, isooxazolyl, thiadiazolyl, oxadiazolyl, thiophenyl, furanyl, quinolinyl, isoquinolinyl, benzimidazolyl, benzoxazolyl, and quinoxalinyl,
each heterocyclyl is selected from the group consisting of 1,3-dioxolane, pyrrolidinyl, pyrazolinyl, pyrazolidinyl, imidazolinyl, imidazolidinyl, piperidinyl, piperazinyl, oxazolidinyl, isoxazolidinyl, morpholinyl, thiazolidinyl, isothiazolidinyl, 2-azabicyclo[2.2.1]-heptyl, 8-azabicyclo[3.2.1]octyl, 5-azaspiro[2.5]octyl, 7-oxooxepan-4-yl, tetrahydropyranyl, and tetrahydrofuryl, and
each optionally substituted group is optionally substituted with 1 or more substituents independently selected from the group consisting of halogen, C1-C4-alkyl, halo-C1-C4-alkyl, C2-C4-alkenyl, halo-C2-C4-alkenyl, C3-C6-cycloalkyl, C1-C4-alkoxy, halo-C1-C4-alkoxy, —CN, OH, NH2, C1-C4-alkylamino, di(C1-C4-alkyl)amino, and NO2.
|