| CPC C07C 17/206 (2013.01) [B01D 61/027 (2013.01); B01D 67/0011 (2013.01); B01D 67/0016 (2013.01); B01D 67/0093 (2013.01); B01D 69/12 (2013.01); B01D 71/021 (2013.01); B01D 71/34 (2013.01); B01D 71/64 (2013.01); B01D 71/68 (2013.01); B01J 23/26 (2013.01); B01J 23/745 (2013.01); B01J 31/0258 (2013.01); B01J 2231/20 (2013.01)] | 10 Claims |
|
1. A continuous preparation method of 2,3,3,3-tetrafluoropropene, comprising the following steps:
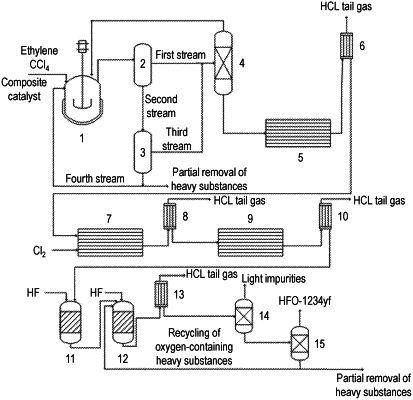
step 1, in the presence of a composite catalyst, continuously introducing ethylene and carbon tetrachloride into a telomerization reactor for liquid-phase catalytic telomerization reaction to obtain a first reaction product, wherein the reaction is carried out at a temperature of 90° C. to 120° C., under a pressure of 0.5 Mpa to 1.0 Mpa and with a residence time of 1 h to 2 h, a molar ratio of carbon tetrachloride to ethylene is (1-4):1, and a mass ratio of carbon tetrachloride to composite catalyst is (40-100):1;
step 2, introducing the first reaction product obtained in the step 1 into a first membrane separator for separation to obtain a first stream and a second stream;
step 3, introducing the second stream obtained in the step 2 into a second membrane separator for separation to obtain a third stream and a fourth stream, and recycling the fourth stream to the telomerization reactor;
step 4, mixing the third stream obtained in the step 3 with the first stream obtained in the step 2, and then introducing the mixture into a first separation column to obtain an overhead fraction of the first separation column and a bottom component of the first separation column, and recycling the overhead fraction of the first separation column to the telomerization reactor;
step 5, carrying out high-temperature cracking and condensing on the bottom component of the first separation column obtained in the step 4 to obtain a second reaction product, wherein the cracking is carried out at a temperature of 350° C. to 500° C., and the residence time is within a range of 1 s to 8 s;
step 6, carrying out gas-phase chlorination and condensing on the second reaction product obtained in the step 5 in the presence of chlorine gas to obtain a third reaction product, wherein the gas-phase chlorination is carried out at a temperature of 140° C. to 170° C., the residence time is within a range of 0.5 s to 5.5 s, and a molar ratio of the chlorine gas to the second reaction product is (1.0-4):1;
step 7, carrying out high-temperature cracking and condensing on the third reaction product obtained in the step 6 to obtain a fourth reaction product, wherein the cracking is carried out at a temperature of 350° C. to 500° C., and the residence time is within a range of 2 s to 15 s;
step 8, in the presence of a first fluorination catalyst, introducing the fourth reaction product obtained in the step 7 and hydrogen fluoride into a first catalytic reactor for gas-phase catalytic fluorination reaction to obtain a fifth reaction product, wherein the reaction is carried out at a temperature of 250° C. to 300° C., under a pressure of 0.5 Mpa to 1.5 Mpa, with a contact time of 1 s to 25 s, and a molar ratio of the HF to the fourth reaction product is (5-25):1; and
step 9, in the presence of a second fluorination catalyst, introducing the fifth reaction product obtained in the step 8 and hydrogen fluoride into a second catalytic reactor for gas-phase catalytic fluorination reaction, wherein the reaction is carried out at a temperature of 250° C. to 330° C., under a pressure of 0.8 Mpa to 1.2 Mpa, with a contact time of 5 s to 35 s, and a molar ratio of the HF to the fifth reaction product is (5-30):1; and condensing and rectifying the reaction product to obtain chlorine-containing column bottom liquid and a 2,3,3,3-tetrafluoropropene product.
|