|
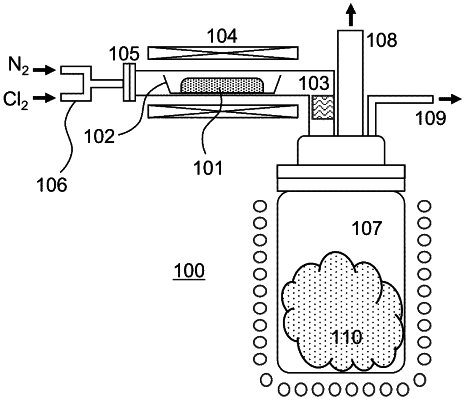
1. A method of producing a molybdenum oxychloride comprising the steps of retaining a gaseous raw material molybdenum oxychloride, which was synthesized based on a reaction of a molybdenum oxide powder and chlorine gas at 700° C. or higher in a vapor phase with inert gas as a carrier gas, at a temperature range of 40° C. or higher and 100° C. or less in an atmospheric pressure, growing crystals of molybdenum oxychloride from the gaseous raw material, and obtaining the crystals of molybdenum oxychloride having a bulk density in an inert atmosphere, wherein the bulk density of the crystals of molybdenum oxychloride is 1.0 g/cm3 or more and a purity of the crystals of molybdenum oxychloride is 99.999 wt % (5N) or higher.
|