| CPC B01D 53/62 (2013.01) [B01D 53/025 (2013.01); B01D 53/1406 (2013.01); B01D 53/1412 (2013.01); B01D 53/1475 (2013.01); B01D 53/1493 (2013.01); C02F 1/001 (2013.01); C02F 1/20 (2013.01); C02F 1/281 (2013.01); C02F 1/66 (2013.01); C02F 1/688 (2013.01); C02F 5/083 (2013.01); B01D 2252/103 (2013.01); B01D 2253/106 (2013.01); B01D 2253/1124 (2013.01); B01D 2253/304 (2013.01); B01D 2257/504 (2013.01); C02F 2209/06 (2013.01); C02F 2209/07 (2013.01); C02F 2209/11 (2013.01); C02F 2209/24 (2013.01)] | 20 Claims |
|
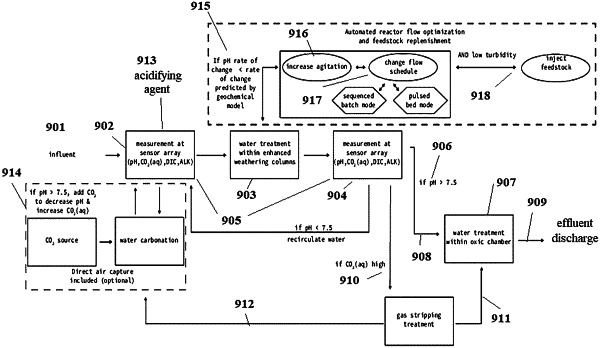
1. A method of at least partially sequestering CO2 from a gaseous CO2 source, the method comprising:
(a) providing an optionally compressed gas stream comprising CO2 at a concentration ranging from about 1% (v/v) to about 100% (v/v);
(b) feeding the optionally compressed gas stream into an influent aqueous solution to provide a second influent aqueous solution comprising CO2;
(c) measuring in the second influent aqueous solution at least two parameters selected from the group consisting of pH, alkalinity, dissolved CO2 concentration, dissolved inorganic carbon (DIC) concentration, bicarbonate ion concentration, carbonate ion concentration, and partial pressure of CO2 (g);
(d) feeding the second influent aqueous solution into at least one container comprising a mineral feedstock, wherein the mineral feedstock is selected from the group consisting of a metal silicate, a metal carbonate, and a metal oxide, and combinations thereof;
(e) contacting the second influent aqueous solution and the mineral feedstock in the container to form an effluent aqueous solution;
measuring in the effluent aqueous solution at least two parameters selected from the group consisting of pH, alkalinity, dissolved CO2 concentration, dissolved inorganic carbon (DIC) concentration, bicarbonate ion concentration, carbonate ion concentration, and partial pressure of CO2 (g); and
(g) comparing the at least two measured parameters of the second influent aqueous solution and the at least two measured parameters of the effluent aqueous solution to calculate a change in dissolved CO2 concentration; and
(h) modifying at least one parameter of the influent aqueous solution or contacting step if the change comprises a decrease in dissolved CO2 concentration of less than about 95%.
|