| CPC A61M 27/006 (2013.01) [A61B 17/0057 (2013.01); A61F 2/01 (2013.01); A61F 2250/0051 (2013.01); A61M 27/002 (2013.01); A61M 2202/0464 (2013.01); A61M 2205/0238 (2013.01); A61M 2205/0266 (2013.01); A61M 2205/04 (2013.01); A61M 2205/3334 (2013.01)] | 22 Claims |
|
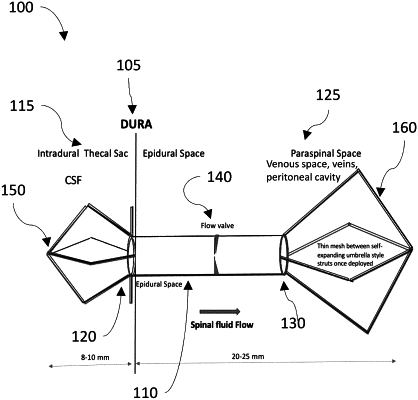
1. An apparatus for percutaneous spinal delivery of a device, the apparatus comprising:
a needle sized to percutaneously inject the device into the spine of the subject, the needle comprising a hollow needle shaft at least partially containing the device, wherein the device comprises:
an implantable structure defining a conduit therein connecting a first end to a second end;
a flow controller positioned within the implantable structure in an orientation that allows spinal fluid to flow through the conduit from the first end to the second end;
a first expansion apparatus positioned at the first end of the implantable structure, wherein the first expansion apparatus is expandable into an expanded configuration larger than the first end; and
a second expansion apparatus positioned at the second end of the implantable structure, wherein the second expansion apparatus is expandable into an expanded configuration larger than the second end.
|