| CPC A61L 2/08 (2013.01) [A01N 35/02 (2013.01); A01N 47/44 (2013.01); A01N 55/02 (2013.01); A01N 55/08 (2013.01); A01N 57/34 (2013.01); A61L 2/18 (2013.01); C07C 217/08 (2013.01); C07C 217/18 (2013.01); C07C 217/20 (2013.01); C07C 217/22 (2013.01); C07C 217/24 (2013.01); C07C 225/06 (2013.01); C07C 279/08 (2013.01); C07F 3/06 (2013.01); C07F 5/022 (2013.01); C07F 9/5407 (2013.01); C07F 9/5442 (2013.01)] | 9 Claims |
|
1. A method for the inactivation of microorganisms, wherein the method comprises the following steps:
(A) bringing the microorganisms into contact with at least one photosensitizer, wherein the photosensitizer is at least one 1,7-diaryl-1,6-heptadiene-3,5-dione derivative with formula (100):
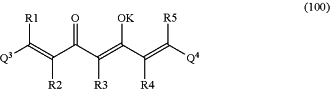 and/or with formula (101):
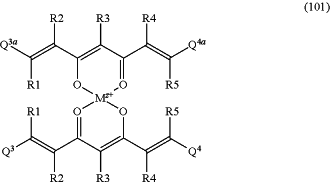 and/or with formula (102):
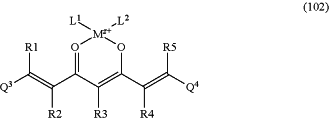 or respectively a pharmacologically acceptable salt and/or ester and/or complex thereof,
wherein the residues Q3, and Q3a, respectively independently of each other,
represent an aromatic residue with the general formula (11a), (12a) or (13a)
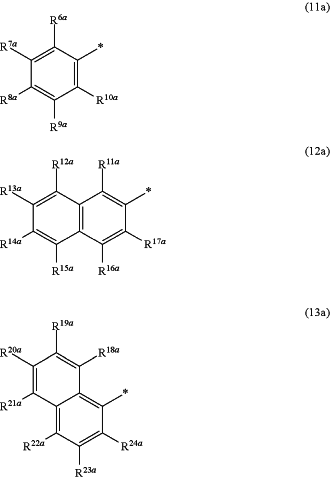 and the residues Q4 and Q4a, respectively independently of each other, represent an aromatic residue with the general formula (11b), (12b) or (13b),
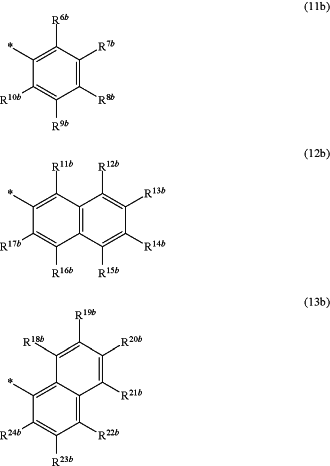 wherein respectively, at least one residue R6a to R10a, R11a to R17a, R18a to R24a, R6b to R10b, R11b to R17b or R18b to R24b, respectively independently of each other, is an organic residue W1, and
wherein none of the residues R6a to R10a, R11a to R17a, R18a to R24a, R6b to R10b, R11b to R17b and R18b to R24b is an organic residue W1,
wherein the residues R6a to R10a, R11a to R17a, R18a to R24a, R6b to R10b, R11b to R17b and R18b to R24b, respectively independently of each other, are identical or different and represent hydrogen, halogen, thiol, nitro, carboxylate, aldehyde containing 1 to 8 C atoms, ketone containing 2 to 8 C atoms, O-alkyl containing 1 to 12 C atoms S-alkyl containing 1 to 12 C atoms O-alkenyl containing 2 to 12 C atoms, S-alkenyl containing 2 to 12 C atoms, O-aryl containing 5 to 20 C atoms, S-aryl containing 5 to 20 C atoms, ether containing 2 to 12 C atoms, thioether containing 2- to 12 C atoms, carboxylic acid ester containing 1 to 12 C atoms, carboxylic acid amide containing 1 to 12 C atoms, thioester containing 1 to 12 C atoms, alkyl containing 1 to 12 C atoms, alkenyl containing 2 to 12 C atoms, cycloalkyl containing 3 to 12 C atoms, cycloalkenyl containing 3 to 12 C atoms, alkylaryl containing 1 to 12 C atoms, aryl containing 5 to 20 C atoms, or heteroaryl, which does not contain a nitrogen atoms, containing 4 to 20 C atoms, and
wherein in formula (100) K represents hydrogen or a cation, and wherein in formula (101) and (102) M2+ is a cation of a metal M or boron, wherein z is the formal oxidation number of the metal M or boron and is a whole number from 1 to 7, and
wherein L1 and L2, respectively independently of each other, represent water, halide, cyanide, thiocyanate, phosphate, hydrogen phosphate, or a carboxylate ion of a carboxylic acid containing 1 to 10 carbon atoms and wherein
(a) at least one of the residues Q3, Q3a, Q4 and Q4a, respectively independently of each other, is substituted with at least one organic residue W1, wherein the at least one organic residue W1 has the general formula (4), (5), (6), (7), (8), or (9):
—(C(D)(E))h-X, (4)
-A-(C(D)(E))h-X, (5)
—(C(D)(E))k-aryl-(C(D)(E))l-X, (6)
-A-(C(D)(E))k-aryl-(C(D)(E))l-X, (7)
—((C(D)(E))m-A)p-(C(D)(E))n-X, (8)
-A-((C(D)(E))m-A)p-(C(D)(E))n-X, (9)
wherein h represents a whole number from 1 to 20, wherein k represents a whole number from 0 to 10, wherein 1 represents a whole number from 0 to 10, and wherein m, n and p, respectively independently of each other, represent a whole number from 1 to 6, and
wherein A, respectively independently of each other, represents oxygen or sulphur,
wherein D and E, respectively independently of each other, represent hydrogen, halogen, G-R(I), or G-C(=G)-R(II), wherein G, respectively independently of each other, represents oxygen or sulphur, and wherein the residues R(I) and R(II), respectively independently of each other, represent hydrogen, methyl, ethyl, n-propyl, n-butyl, n-pentyl, phenyl or benzyl, wherein phenyl and benzyl may be unsubstituted or substituted,
wherein aryl represents a substituted or unsubstituted aromatic group or a substituted or unsubstituted heteroaromatic group which does not contain a nitrogen atom,
wherein X, respectively independently of each other, is an organic residue which (i) contains at least one neutral nitrogen atom which can be protonated, or (ii) contains at least one positively charged nitrogen atom, or (iii) contains at least one positively charged phosphorus atom, each having independently at least one counter-ion or a mixture of two or more counter-ions selected from a fluoride, a chloride, a bromide, an iodide, a sulphate, a hydrogen sulphate, a phosphate, a hydrogen phosphate, a dihydrogen phosphate, a tosylate, mesylate, and at least one carboxylate ion of a carboxylic acid containing 1 to 15 carbon atoms, and
wherein the residues R1, R2, R3, R4 and R5, respectively independently of each other, represent hydrogen, halogen, alkyl containing 1 to 12 C atoms, cycloalkyl containing 1 to 12 C atoms, alkylaryl containing 1 to 12 C atoms, aryl containing 5 to 20 C atoms, ether containing 2 to 12 C atoms or glycol containing 2 to 12 C atoms,
or wherein
(b) the residue R3 is an organic residue W2, wherein the one organic residue W2 has the general formula (4), (5), (6), (7), (8), (9), or (10):
—(C(D)(E))h-X, (4)
-A-(C(D)(E))h-X, (5)
—(C(D)(E))k-aryl-(C(D)(E))l-X, (6)
-A-(C(D)(E))k-aryl-(C(D)(E))l-X, (7)
—((C(D)(E))m-A)p-(C(D)(E))n-X, (8)
-A-((C(D)(E))m-A)p-(C(D)(E))n-X, (9),
—(—C(D)=C(E)-)r-X, (10),
and
wherein, optionally, at least one of the residues Q3, Q3a, Q4 and Q4a, respectively independently of each other, is substituted with at least one organic residue W1 which has the general formula (4), (5), (6), (7), (8), or (9),
wherein h represents a whole number from 1 to 20, wherein k represents a whole number from 0 to 10, wherein 1 represents a whole number from 0 to 10, and wherein m, n, p, and r, respectively independently of each other, represent a whole number from 1 to 6, and
wherein A, respectively independently of each other, represents oxygen or sulphur,
wherein D and E, respectively independently of each other, represent hydrogen, halogen, G-R(I), or G-C(=G)-R(II), wherein G, respectively independently of each other, represents oxygen or sulphur, and wherein the residues R(I) and R(II), respectively independently of each other, represent hydrogen, methyl, ethyl, n-propyl, n-butyl, n-pentyl, phenyl or benzyl, wherein phenyl and benzyl may be unsubstituted or substituted,
wherein aryl represents a substituted or unsubstituted aromatic group or a substituted or unsubstituted heteroaromatic group which does not contain a nitrogen atom,
wherein X, respectively independently of each other, is an organic residue which (i) contains at least one neutral nitrogen atom which can be protonated, or (ii) contains at least one positively charged nitrogen atom, or (iii) contains at least one positively charged phosphorus atom, each having independently at least one counter-ion or a mixture of two or more counter-ions selected from a fluoride, chloride, bromide iodide, sulphate, hydrogen sulphate, phosphate hydrogen phosphate, dihydrogen phosphate, tosylate, mesylate, and at least one carboxylate ion of a carboxylic acid containing 1 to 15 carbon atoms, and
wherein the residues R1, R2, R4 and R5, respectively independently of each other, represent hydrogen, halogen, alkyl containing 1 to 12 C atoms, alkylaryl containing 1 to 12 C atoms, aryl containing 5 to 20 C atoms, ether containing 2 to 12 C atoms or glycol containing 2 to 12 C atoms, and
(B) irradiating the microorganisms and the at least one photosensitizer with electromagnetic radiation of a suitable wavelength and energy density.
|