| CPC A61K 51/0485 (2013.01) [A61K 47/545 (2017.08); A61K 51/0478 (2013.01); A61K 51/0482 (2013.01)] | 3 Claims |
|
1. A low molecular weight compound of Formula (I):
B-L-A (I)
wherein:
A is a targeting moiety for FAP-α, wherein A has the structure of:
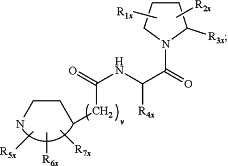 wherein:
R1x and R2x are each independently selected from the group consisting of H, OH, halogen, C1-6alkyl, —O—C1-6alkyl, and —S—C1-6alkyl;
R3x is selected from the group consisting of H, —CN, —B(OH)2, —C(O)alkyl, —C(O)aryl-, —C═C—C(O)aryl, —C═C—S(O)2aryl, —CO2H, —SO3H, —SO2NH2, —PO3H2, and 5-tetrazolyl;
R4x is H;
R5x, R6x, and R7x are each independently selected from the group consisting of H, —OH, oxo, halogen, —C1-6alkyl, —O—C1-6alkyl, —S—C1-6alkyl, —NR8xR9x, —OR12x, —Het2 and —Ar2 each of C1-6 alkyl being optionally substituted with from 1 to 3 substituents selected from —OH and halogen;
R8x, R9x, and R12x are each independently selected from the group consisting of H, —OH, halo, —C1-6alkyl, —O—C1-6alkyl, —S—C1-6alkyl, and —Ar3;
R10x, R11x, R13x and R14x are each independently selected from the group consisting of H, —OH, halogen, —C1-6alkyl, —O—C1-6alkyl, and —S—C1-6alkyl; Ar1, Ar2 and Ar3 are each independently a 5- or 6-membered aromatic monocycle optionally comprising 1 or 2 heteroatoms selected from O, N and S; each of Ar1, Ar2 and Ar3 being optionally and independently substituted with from 1 to 3 substituents selected from —NR10xR11x, —C1-6alkyl, —O—C1-6alkyl, and —S—C1-6alkyl;
Het2 is a 5- or 6-membered non-aromatic monocycle optionally comprising 1 or 2 heteroatoms selected from O, N and S; Het2 being optionally substituted with from 1 to 3 substituents selected from —NR13xR14x, —C1-6alkyl, —O—C1-6alkyl, and —S—C1-6alkyl;
v is 0, 1, 2, or 3; and
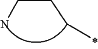 represents a 5 to 10-membered N-containing aromatic or non-aromatic mono- or bicyclic heterocycle, said heterocycle optionally further comprising 1, 2 or 3 heteroatoms selected from O, N and S;
B is any optical or radiolabeled functional group suitable for optical imaging, positron-emission tomography (PET) imaging, single-photon emission computed tomography (SPECT) imaging, or radiotherapy; and L is a linker having bi-functionalization adapted to form a chemical bond with B and A; or
a stereoisomer, tautomer, racemate, salt, hydrate, or solvate thereof.
|