| CPC A61K 49/0013 (2013.01) [C07D 487/04 (2013.01); G01N 21/763 (2013.01); G01N 33/573 (2013.01)] | 4 Claims |
|
1. A kit comprising:
i) A compound of following formula:
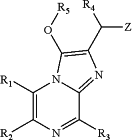 wherein:
R1 represents H or a group selected from C1-C6 alkyl, C3-C7 cycloalkyl, C6-C10 aryl, aralkyl and C5-C10-membered heteroaryl groups, said C1-C6 alkyl, C3-C7 cycloalkyl, C6-C10 aryl, aralkyl and C5-C10-membered heteroaryl groups are optionally substituted by at least one Y1 group;
R2 represents a group selected from C6-C10 aryl and C5-C10-membered heteroaryl groups, said C6-C10 aryl and C5-C10-membered heteroaryl groups are optionally substituted by at least one Y2 group;
or R1 and R2 together form with the two carbon atom to which they are respectively attached a C5-C7 cycloalkene group, a C4-C7 heterocycloalkene group, or a C6-C10 arene, said C5-C7 cycloalkene group and C4-C7 heterocycloalkene groups are fused with a C6-C10 arene, said C5-C7 cycloalkene group, C4-C7 heterocycloalkene group, C6-C10 arene, C5-C7 cycloalkene group and C4-C7 heterocycloalkene groups are optionally substituted by at least one Y12 group;
R3 represents H, a C1-C6 alkyl, an aralkyl group, a hetaralkyl group or a heterocycloalkyl-CH2— group, said C1-C6 alkyl, aralkyl group, hetaralkyl group and heterocycloalkyl-CH2— group are optionally substituted by at least one Y3 group;
R4 represents H or a group selected from C1-C6 alkyl and C3-C7 cycloalkyl groups, said C1-C6 alkyl and C3-C7 cycloalkyl groups are optionally substituted by at least one Y4 group;
R5 represents a —C(═O)Ra group or a —C(═O)ORa group, said C(═O)Ra group and —C(═O)ORa group are optionally substituted by at least one Y5 group;
Ra represents H, a C1-C6 alkyl group, a C3-C7 cycloalkyl group, a C6-C10 aryl, or an aralkyl group, said C1-C6 alkyl group, C3-C7 cycloalkyl group, C6-C10 aryl, and aralkyl group are optionally substituted by at least one Ya group;
Z represents a C6-C10 aryl, a C5-C10-membered heteroaryl groups, a C1-C6 alkyl, a C3-C7 cycloalkyl, a C4-C7 heterocycloalkyl, said C6-C10 aryl, C5-C10-membered heteroaryl groups, C1-C6 alkyl, C3-C7 cycloalkyl and C4-C7 heterocycloalkyl are optionally substituted by at least one YZ group;
said Y1, Y2, Y12, Y3, Y4, Y5, Ya and YZ groups are each independently selected from:
a C1-C6 alkyl;
a C3-C7 cycloalkyl;
a C6-C10 aryl;
a C5-C10-membered heteroaryl group;
an halogen;
a —CF3 group;
a —CN group;
a —ORi group;
a —OSO3H group;
a —NRiRii group;
a guanidinyl group;
a —C(═O)ORa group, Ra is as defined above;
Ri and Rii each independently represent H, a C1-C6 alkyl group, a C3-C10 cycloalkyl group, an aralkyl group; or together form with the nitrogen atom to which they are attached a C4-C7 heterocycloalkyl group;
and
ii) a solution comprising a strong acid.
|