| CPC A61K 47/6803 (2017.08) [A61K 31/5517 (2013.01); A61K 47/6849 (2017.08); A61K 47/6867 (2017.08); A61P 35/02 (2018.01); C07K 16/2827 (2013.01); A61K 9/0019 (2013.01); A61K 9/0053 (2013.01); A61K 31/519 (2013.01); A61K 31/573 (2013.01); A61K 31/7068 (2013.01); A61K 2039/505 (2013.01); A61K 2039/542 (2013.01); A61K 2039/545 (2013.01); C07K 16/2887 (2013.01)] | 19 Claims |
|
1. A method of treating a subject suffering from a proliferative disease, said method comprising:
administering to a subject suffering from a proliferative disease an effective amount of a CD19-antibody drug conjugate (ADC),
wherein (i) the CD19-ADC comprises as the a pyrrolobenzodiazepine (PBD) dimer, linked to an antibody that binds to CD19; and (ii) the CD19-ADC has a drug to antibody ratio (DAR) of between 1 and 8,
wherein (i) the CD19-ADC is administered to the subject in a dosage regime comprising at least two treatment cycles; and (ii) the dose of CD19-ADC is reduced following the second treatment cycle; and
wherein (i) the proliferative disease is a CD19+ cancer or a CD19+ autoimmune disease; and (ii) the PBD dimer is of formula I:
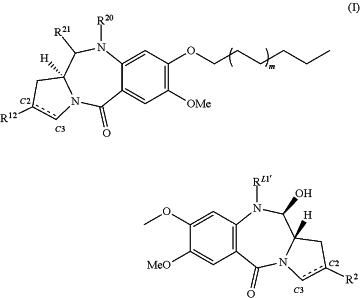 wherein
(a) RL1′ is a linker for connection to Ab, wherein RL1′ is
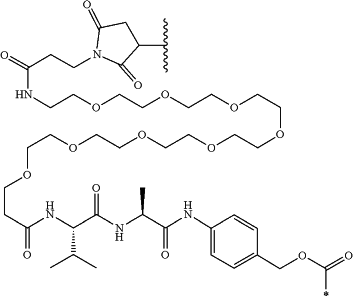 wherein the wavy line indicates the point of attachment to the Ab and the asterisk indicates the point of attachment to the nitrogen in Formula (I);
(b) (i) R20 and R21 either together form a double bond between the nitrogen and carbon atoms to which they are bound; or (ii) R20 is a capping group RC, and R21 is OH;
(c) m is 0 or 1; and
(d) when there is a double bond between C2 and C3, R2 is methyl;
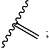 when there is a single bond between C2 and C3, R2 is either H or
when there is a double bond between C2′ and C3′, R12 is methyl;
when there is a single bond between C2′ and C3′, R12 is H or
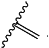 |